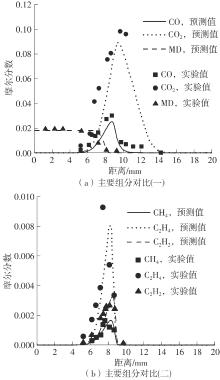
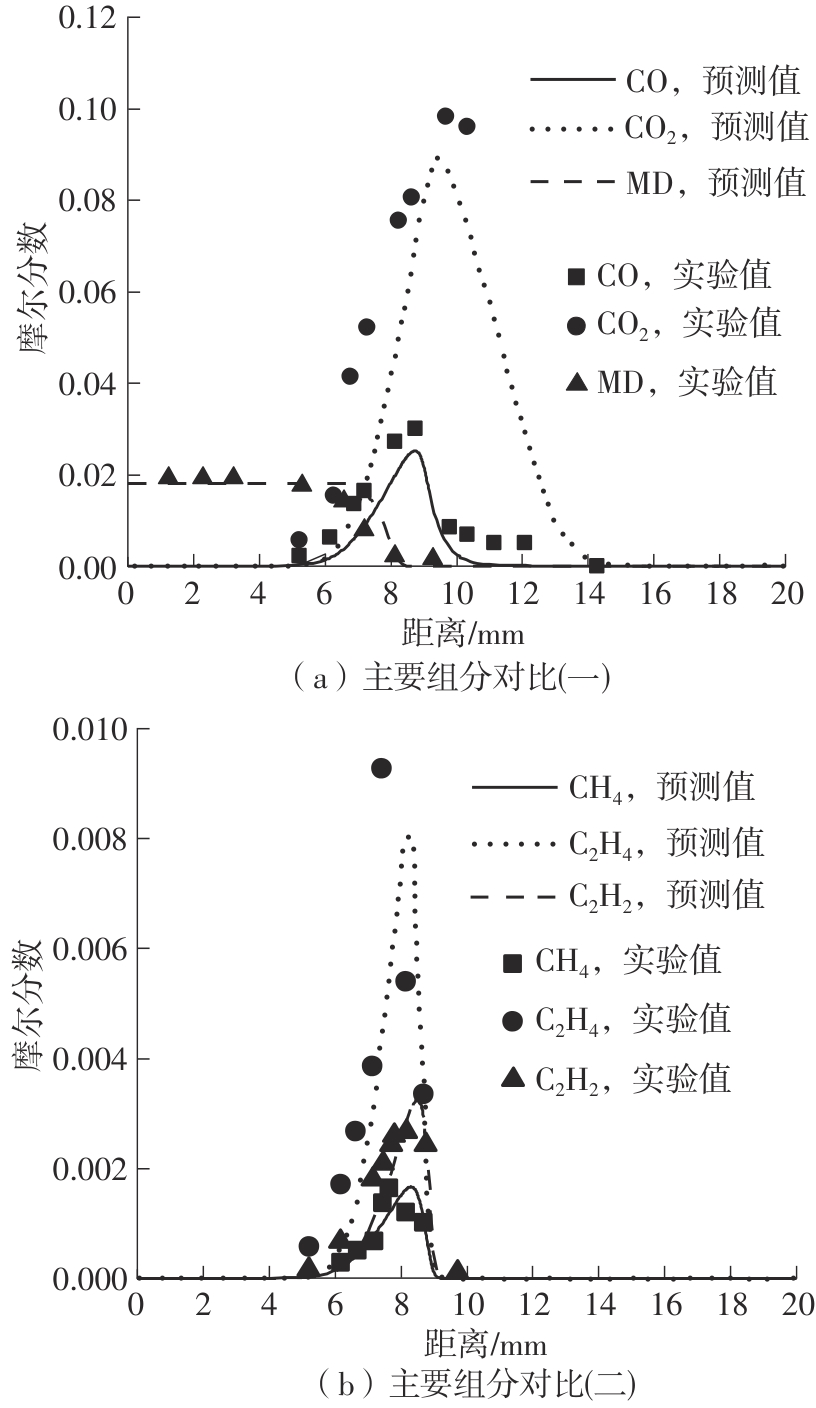
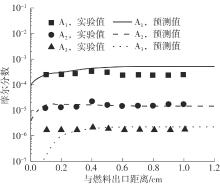
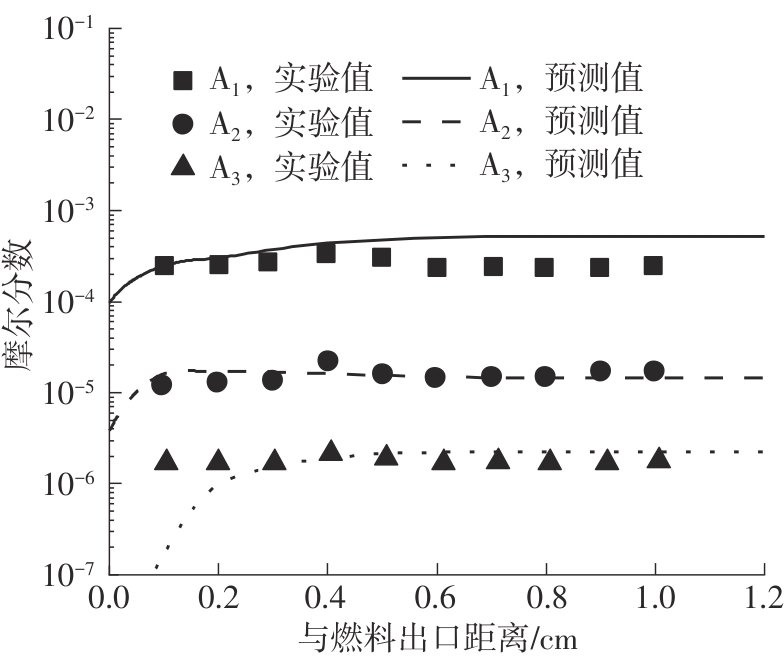
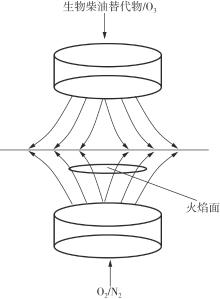
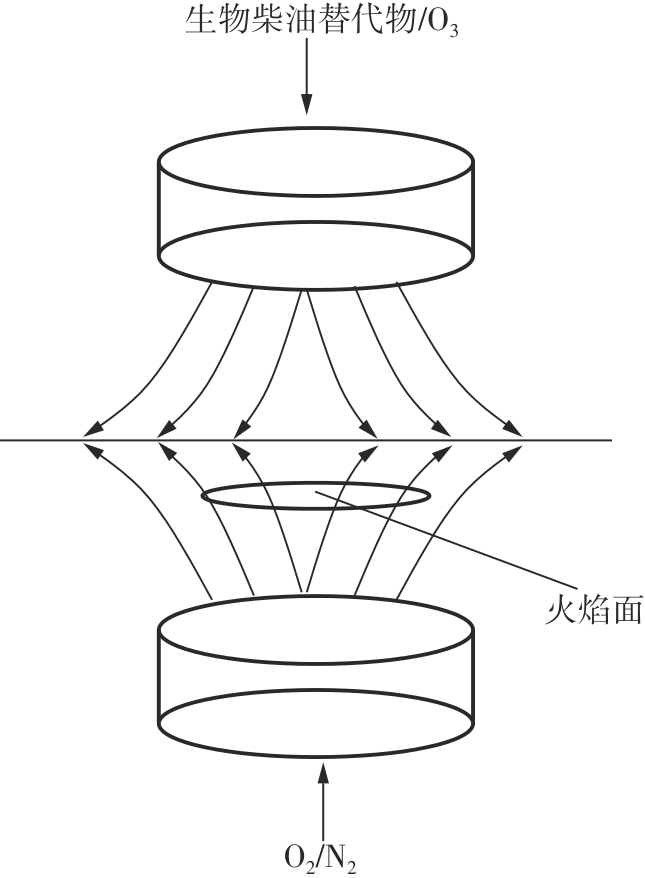
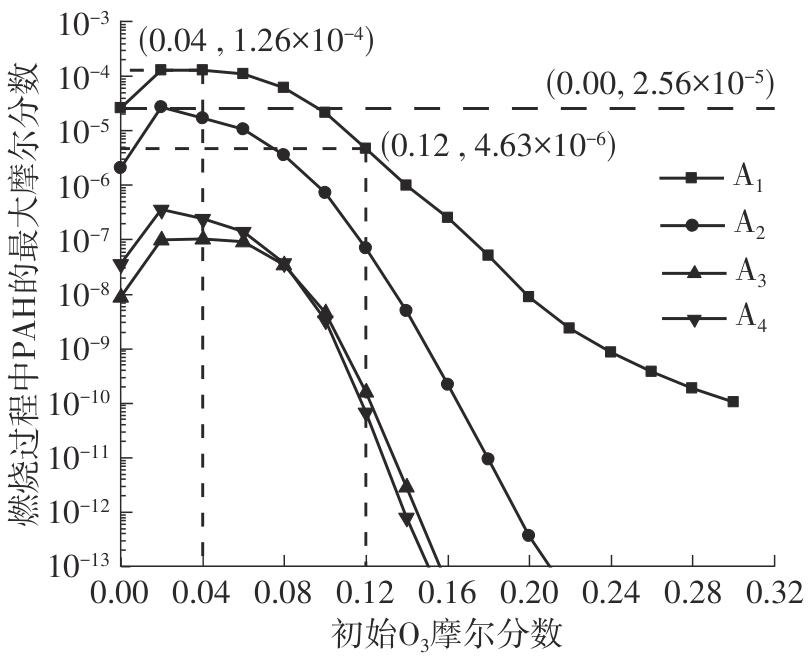
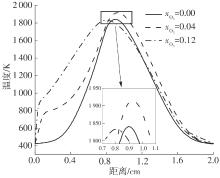
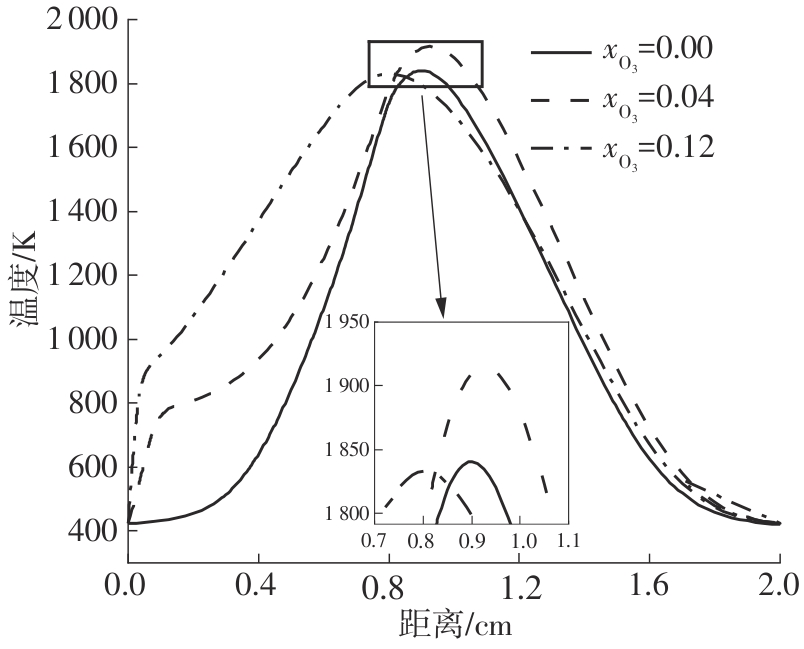
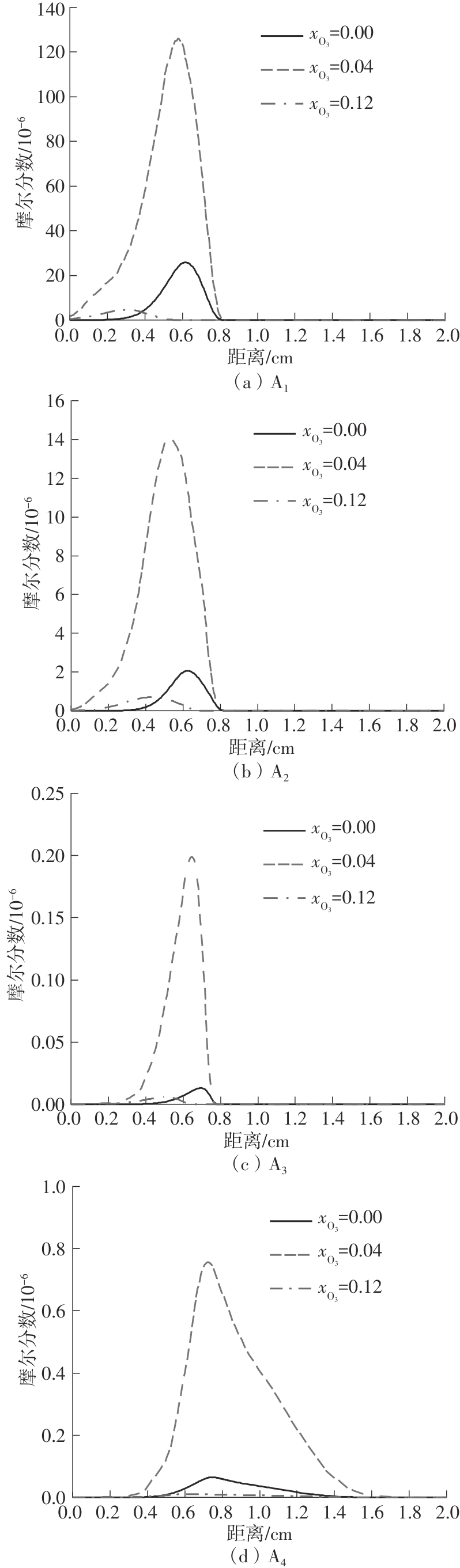
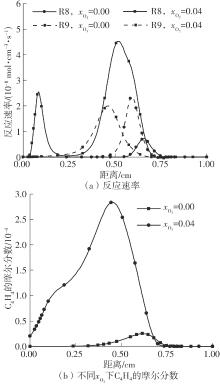
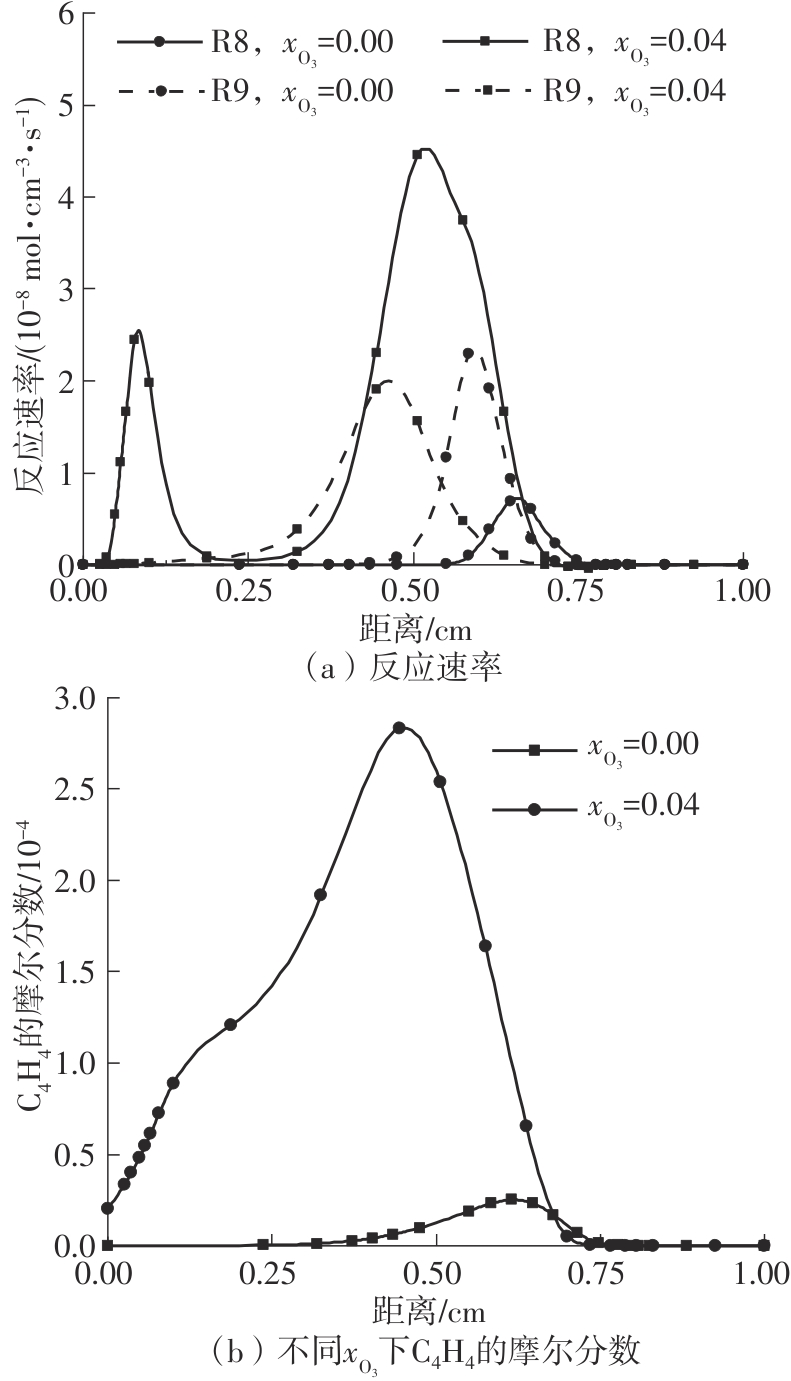
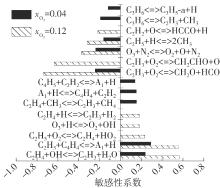
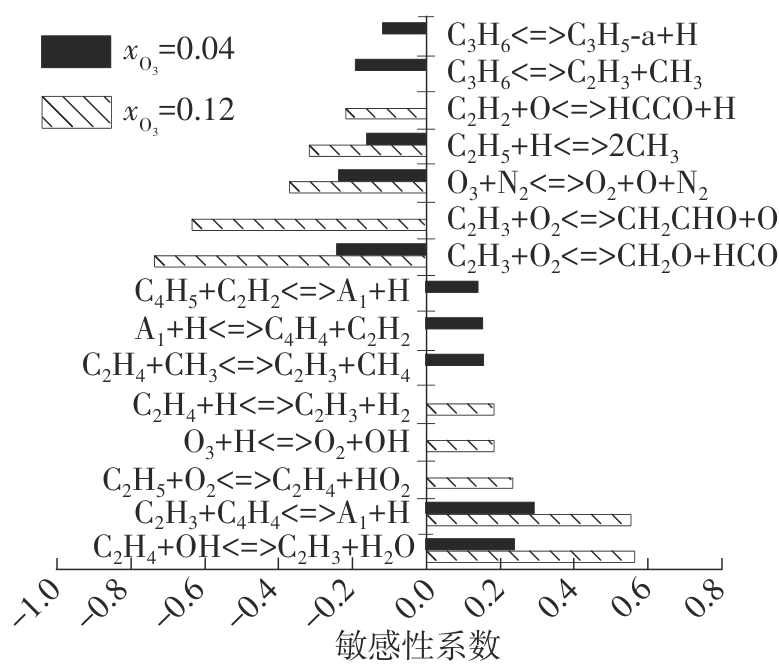
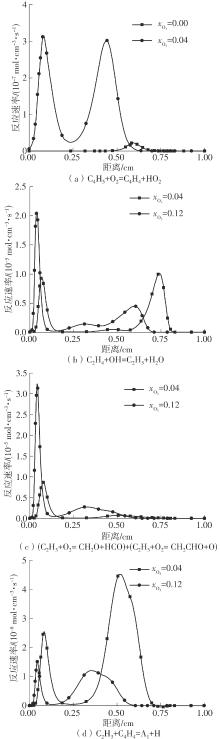
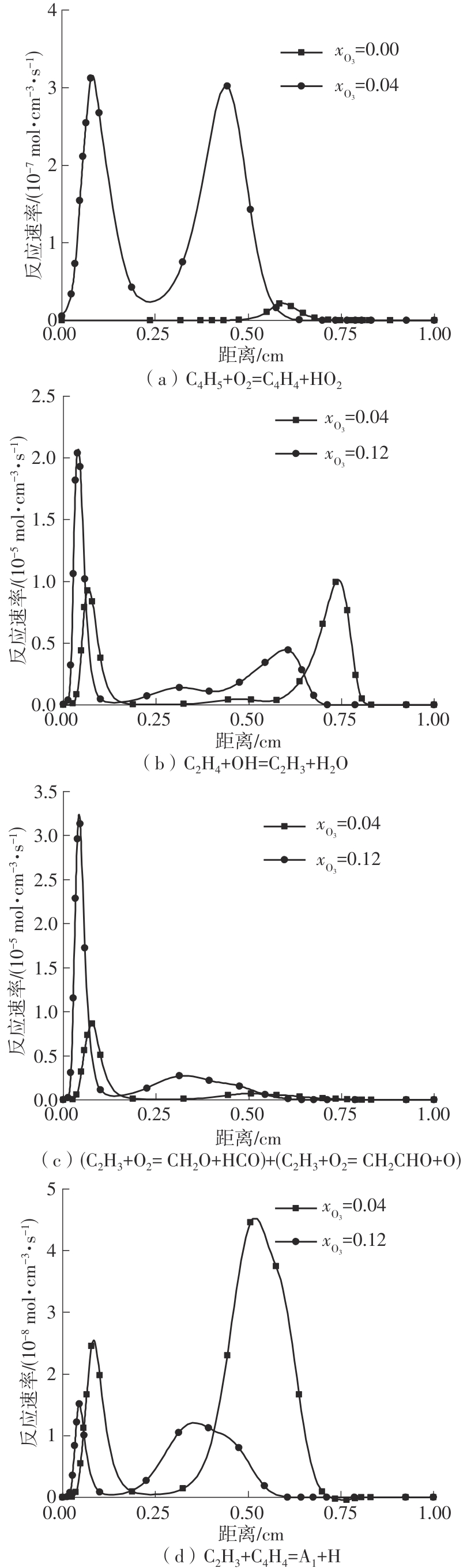
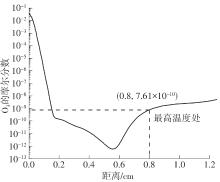
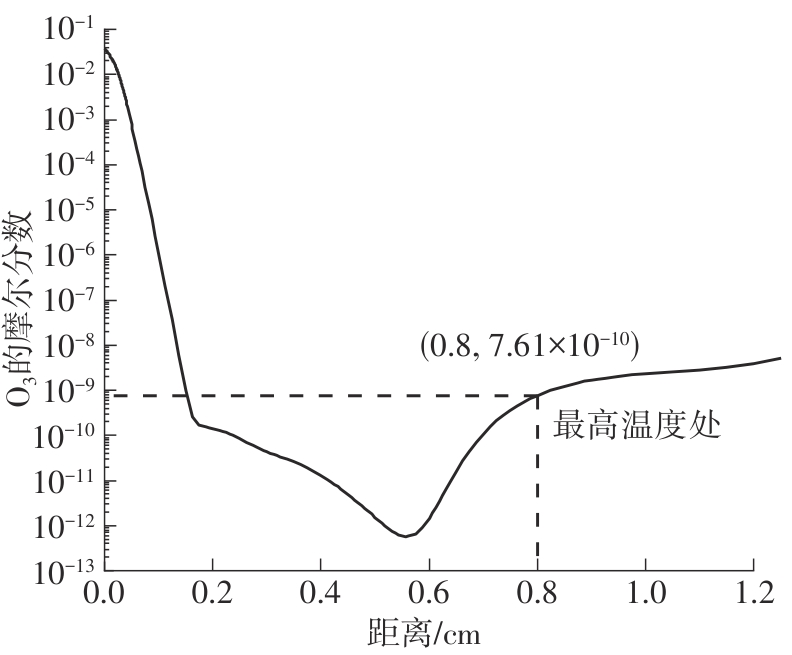
Journal of South China University of Technology(Natural Science Edition) ›› 2025, Vol. 53 ›› Issue (7): 11-20.doi: 10.12141/j.issn.1000-565X.240235
• Energy, Power & Electrical Engineering • Previous Articles Next Articles
Effect of Ozone on Polycyclic Aromatic Hydrocarbon Formation in Combustion of Biodiesel Surrogate
GAN Yunhua1, LIU Zhuolong1, KUANG Hualin2, HAN Yanjie2, LI Hua1
- 1.School of Electric Power Engineering,South China University of Technology,Guangzhou 510640,Guangdong,China
2.Guangzhou Zhongdian Lixin Thermal Power Co. ,Ltd. ,Guangzhou 511340,Guangdong,China
-
Received:2024-05-15Online:2025-07-25Published:2024-12-27 -
About author:甘云华(1979—),男,博士,教授,主要从事新型燃烧、高效传热等研究。E-mail: ganyh@scut.edu.cn -
Supported by:the National Natural Science Foundation of China(52376108);the Science and Technology Planning Project of Guangdong Province(2022A0505050004)
CLC Number:
Cite this article
GAN Yunhua, LIU Zhuolong, KUANG Hualin, HAN Yanjie, LI Hua. Effect of Ozone on Polycyclic Aromatic Hydrocarbon Formation in Combustion of Biodiesel Surrogate[J]. Journal of South China University of Technology(Natural Science Edition), 2025, 53(7): 11-20.
share this article
| [1] | 梅德清,俞玥,高亚平,等 .多组分燃料液滴高温蒸发耦合机制研究[J].华南理工大学学报(自然科学版),2023,51(3):33-40,82. |
| MEI Deqing, YU Yue, GAO Yaping,et al .Coupling mechanism of high-temperature evaporation of multi-component fuel droplets[J].Journal of South China University of Technology (Natural Science Edition),2023,51(3):33-40,82. | |
| [2] | ISSARIYAKUL T, DALAI A K .Biodiesel from vegetable oils[J].Renewable and Sustainable Energy Reviews,2014,31:446-471. |
| [3] | WESTBROOK C K, NAIK C V, HERBINET O,et al .Detailed chemical kinetic reaction mechanisms for soy and rapeseed biodiesel fuels[J].Combustion and Flame,2011,158(4):742-755. |
| [4] | FISHER E M, PITZ W J, CURRAN H J,et al .Detailed chemical kinetic mechanisms for combustion of oxygenated fuels[J].Proceedings of the Combustion Institute,2000,28(2):1579-1586. |
| [5] | HERBINET O, PITZ W J, WESTBROOK C K .Detailed chemical kinetic mechanism for the oxidation of biodiesel fuels blend surrogate[J].Combustion and Flame,2010,157(5):893-908. |
| [6] | LUO Z, PLOMER M, LU T,et al .A reduced mechanism for biodiesel surrogates for compression ignition engine applications[J].Fuel,2012,99:143-153. |
| [7] | NI Z, LI F, WANG H .Simplification of the combustion mechanism of Jatropha biodiesel surrogate fuel and reaction path analysis[J].Energy,2023,282:128859/1-9. |
| [8] | 王晶,张漫 .不同前驱体模型对航空煤油扩散火焰碳烟颗粒生成的影响研究[J].工程热物理学报,2021,42(12):3286-3295. |
| WANG Jing, ZHANG Man .Study on the influence of different soot inception models on the soot particles formation in a aviation kerosene diffusion flame[J].Journal of Engineering Thermophysics,2021,42(12):3286-3295. | |
| [9] | SLAVINSKAYA N A, FRANK P .A modelling study of aromatic soot precursors formation in laminar methane and ethene flames[J].Combustion and Flame,2009,156(9):1705-1722. |
| [10] | WANG H, DENEYS R R, YAO M F,et al .Development of an n-heptane-n-butanol-PAH mechanism and its application for combustion and soot prediction[J].Combustion and Flame,2013,160(3):504-519. |
| [11] | DONG X, CHANG Y, NIU B,et al .Development of a practical reaction model of polycyclic aromatic hydrocarbon (PAH) formation and oxidation for diesel surrogate fuel[J].Fuel,2020,267:117159/1-14. |
| [12] | WU Y X, LIU F X, SUN Y L,et al .Effects of carbon dioxide and water vapor addition on benzene and PAH formation in a laminar premixed CH4/O2/Ar flame[J].Combustion Science and Technology,2019,191(10):1866-1897. |
| [13] | LIU F, DWORKIN S B, THOMSON M J,et al .Modeling DME addition effects to fuel on PAH and soot in laminar coflow ethylene/air diffusion flames using two PAH mechanisms[J].Combustion Science and Technology,2012,184(7/8/9):966-979. |
| [14] | ZENG W .Single zone simulation of polycyclic aromatic hydrocarbon formation in n-heptane HCCI combustion[J].Advanced Materials Research,2011,236/237/238:525-529. |
| [15] | LI S, GAO J, HUANG C,et al .Development of a reduced n-heptane/toluene/unsaturated furans-PAH reaction mechanism for dual-fuel engine applications[J].Fuel,2022,317:123466/1-15. |
| [16] | WANG H, LI Y, IQBAL Z,et al .A comparison study on the combustion and sooting characteristics of base engine oil and n-dodecane in laminar diffusion flames[J].Applied Thermal Engineering,2019,158:113812/1-6. |
| [17] | LIU X L, WANG H, WEI L X,et al .Development of a reduced toluene reference fuel (TRF)-2,5-dimethylfuran-polycyclic aromatic hydrocarbon (PAH) mechanism for engine applications[J].Combustion and Flame,2016,165:453-465. |
| [18] | YAMADA H, YOSHII M, TEZAKI A .Chemical mechanistic analysis of additive effects in homogeneous charge compression ignition of dimethyl ether[J].Proceedings of the Combustion Institute,2005,30(2):2773-2780. |
| [19] | FOUCHER F, HIGELIN P, MOUNAÏM-ROUSSELLE C,et al .Influence of ozone on the combustion of n-heptane in a HCCI engine[J].Proceedings of the Combustion Institute,2013,34(2):3005-3012. |
| [20] | MASURIER J, FOUCHER F, DAYMA G,et al .Investigation of iso-octane combustion in a homogeneous charge compression ignition engine seeded by ozone,nitric oxide and nitrogen dioxide[J].Proceedings of the Combustion Institute,2015,35(3):3125-3132. |
| [21] | SEIGNOUR N, MASURIER J, JOHANSSON B,et al .Ozone-assisted combustion of hydrogen:a comparison with isooctane[J].International Journal of Hydrogen Energy,2019,44(26):13953-13963. |
| [22] | LIU S, LIN Z L, ZHANG H,et al .Impact of ammonia addition on knock resistance and combustion performance in a gasoline engine with high compression ratio[J].Energy,2023,262:125458/1-12. |
| [23] | ZHOU Y, GAN Y H, ZHANG C Y,et al .Numerical study for influence of ozone on the combustion of biodiesel surrogates in a homogeneous charge compression ignition engine[J].Fuel Processing Technology,2022,225:107039/1-12. |
| [24] | SLAVINSKAYA N A, RIEDEL U, DWORKIN S B,et al .Detailed numerical modeling of PAH formation and growth in non-premixed ethylene and ethane flames[J].Combustion and Flame,2012,159(3):979-995. |
| [25] | HALTER F, HIGELIN P, DAGAUT P .Experimental and detailed kinetic modeling study of the effect of ozone on the combustion of methane[J].Energy & Fuels,2011,25:2909-2916. |
| [26] | SARATHY S M, THOMSON M J, PITZ W J,et al .An experimental and kinetic modeling study of methyl decanoate combustion[J].Proceedings of the Combustion Institute,2011,33(1):399-405. |
| [27] | HE Y T, LUO Y, ZHANG Q,et al .Effects of air-side dimethyl ether premixing on combustion characteristics of nonpremixed biodiesel/air counterflow flames[J].Fuel,2022,316:123306/1-11. |
| [28] | INAL F, SENKAN S M .Effects of equivalence ratio on species and soot concentrations in premixed n-heptane flames[J].Combustion and Flame,2002,131(1/2):16-28. |
| [29] | SOLMAZ E, BISETTI F .Flamelet chemistry model for efficient axisymmetric counterflow flame simulations with realistic nozzle geometries and gravitational body force[J].Combustion Theory and Modelling,2020,24(5):926-952. |
| [1] | LOU Bo, ZHOU Daheng, XIA Jun. Kinetics of Dehydration/Adsorption Reaction of LaCl3 [J]. Journal of South China University of Technology(Natural Science Edition), 2023, 51(8): 71-79. |
| [2] | LEI Lirong DANG Zhongxu LI Youming. Catalytic Ozonation of Eugenol in the Presence of Fe2O3 Supported by Activated Carbon#br# [J]. Journal of South China University of Technology(Natural Science Edition), 2018, 46(10): 132-140. |
| [3] | ZHONG Li ZHANG Li HUANG Hong. Structure Characterization and Reaction Kinetics of TDI-TMP Prepolymer [J]. Journal of South China University of Technology (Natural Science Edition), 2017, 45(6): 109-116. |
| [4] | LI You-ming CHEN Jing-tian LEI Li-rong. Effect and Kinetics Analysis of Eugenol Degradation by Ozone [J]. Journal of South China University of Technology (Natural Science Edition), 2016, 44(6): 90-97. |
| [5] | Chen Sai-yan Li You-ming Lei Li-rong. Tertiary Treatment of Paper-Making Tobacco Sheet Wastewater via Catalytic Ozonation with Ti(Ⅳ) Ions#br# [J]. Journal of South China University of Technology (Natural Science Edition), 2015, 43(10): 131-139. |
| [6] | Fan Xiao- jiang Lei Ying Zhang Xi- hui Chao Meng Hiroshi Noguchi. Investigation into a New Integrated Water Purification Process of Half Coagulation/Ozonation/Ceramic Membrane Filtration [J]. Journal of South China University of Technology (Natural Science Edition), 2014, 42(5): 54-59. |
| [7] | Long Xin-feng Wu Juan. Thermal Decomposition Kinetics of Thermochemical Energy Storage System Ca(OH)2 /CaO [J]. Journal of South China University of Technology (Natural Science Edition), 2014, 42(10): 75-81. |
| [8] | Zou Hua-sheng Lei Min Li Jian. Biodiesel Production from Lard Catalyzed by Calcium-Based Solid Base in Ultrasonic Field [J]. Journal of South China University of Technology(Natural Science Edition), 2012, 40(9): 8-13. |
| [9] | Zhang Ling-hui Su Da-gen. Effect of Cement Calcination with Municipal Sewage Sludge on Nitrogen Oxide Emission [J]. Journal of South China University of Technology(Natural Science Edition), 2012, 40(4): 90-94. |
| [10] | Lei Li-rong Li You-ming Ma Li-ming. Pulping Effluent Treatment with Ozone Catalyzed by Activated Carbon and Alumina Loaded or Unloaded with Titanium Dioxide [J]. Journal of South China University of Technology(Natural Science Edition), 2012, 40(2): 149-155. |
| [11] | Luo Yuan-fang Yang Chao Jia Zhi-xin Jia De-min Chen Jun Zheng De. Antiaging Effect of Vitamin C-Rare Earth Complex on Natural Rubber /Carbon Black Composites [J]. Journal of South China University of Technology(Natural Science Edition), 2012, 40(10): 128-133. |
| [12] | LU Zhi-Min. Modeling Experiment of SNCR Injection Based on Heat Balance Theory [J]. Journal of South China University of Technology (Natural Science Edition), 2011, 39(3): 78-82. |
| [13] | Zou Hua-sheng Li Jian Chen Wen-biao. Ultrasonic-Enhanced Preparation of Biodiesel via Transesterification Catalyzed by Solid Base [J]. Journal of South China University of Technology(Natural Science Edition), 2011, 39(11): 6-11. |
| [14] | Ma Yan Gao Nai-yun Yao Juan-juan Guo Hong-guang Zhang Ke-jia . Degradation of Tetracycline Hydrochloride in Aqueous Solution by Ultrasonic Irradiation [J]. Journal of South China University of Technology (Natural Science Edition), 2010, 38(8): 147-152. |
| [15] | Zhang Hui-ping Peng Guan-lan Yan Ying Guan Jian-yu Xiao Xin-yan . Intrinsic Reaction Kinetics CO on Cu-Mn of Catalytic Oxidation of Catalyst Bed [J]. Journal of South China University of Technology (Natural Science Edition), 2009, 37(6): 13-16. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||