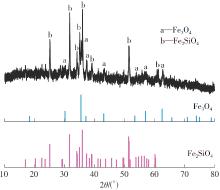
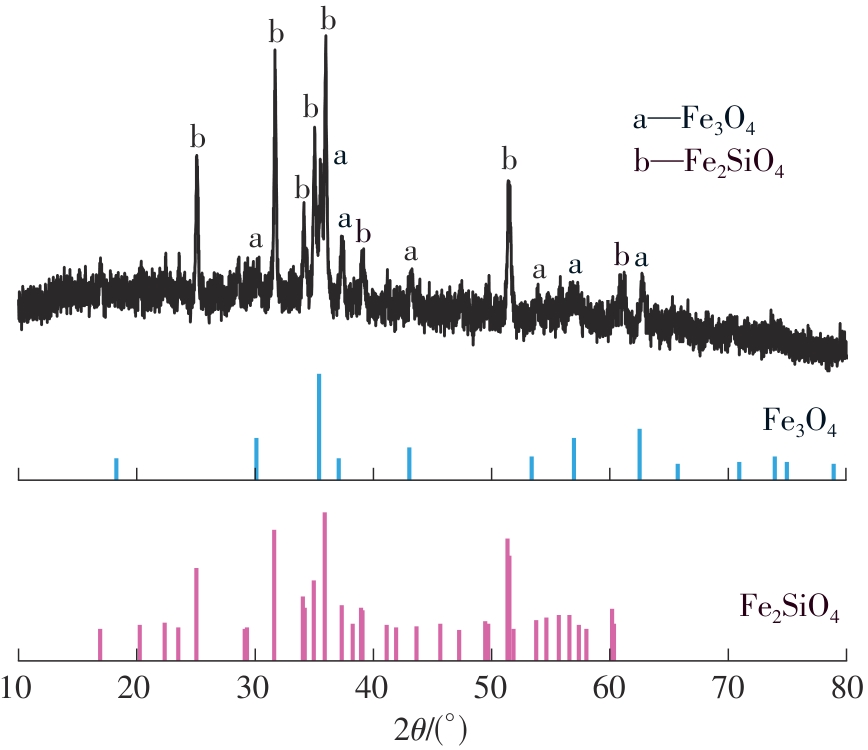
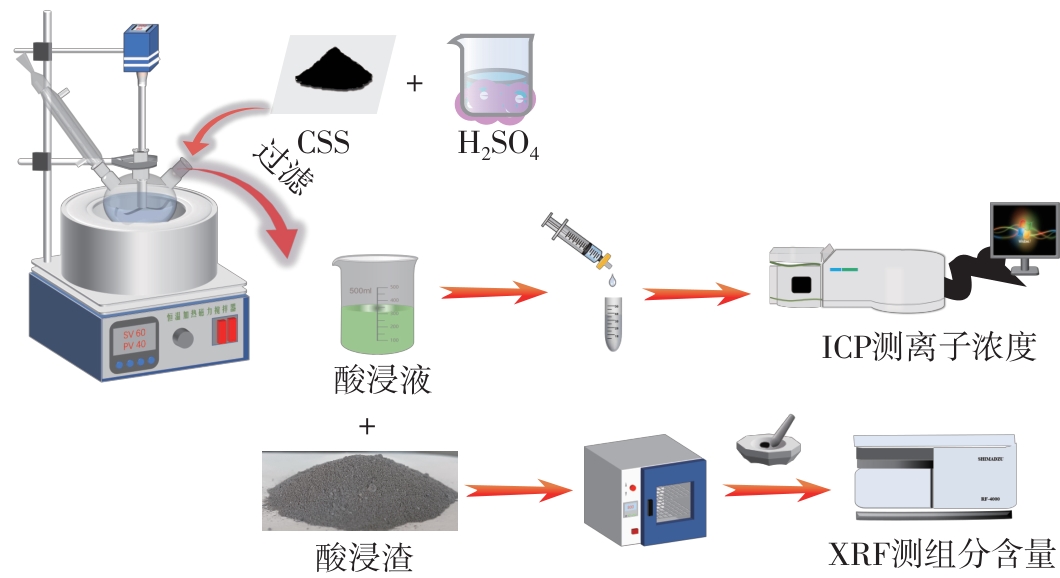
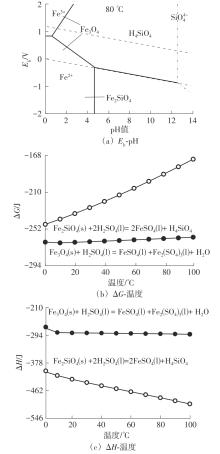
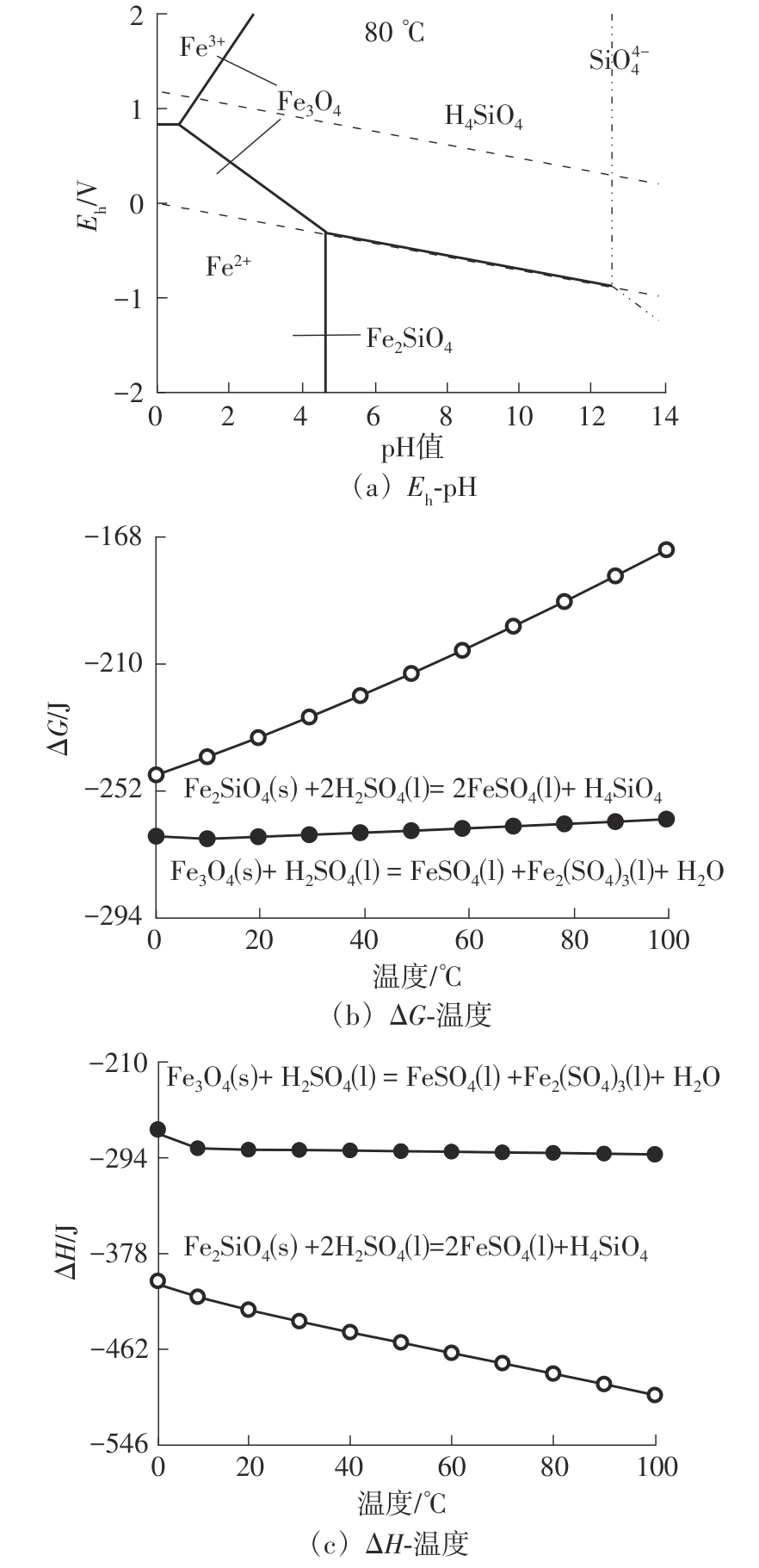
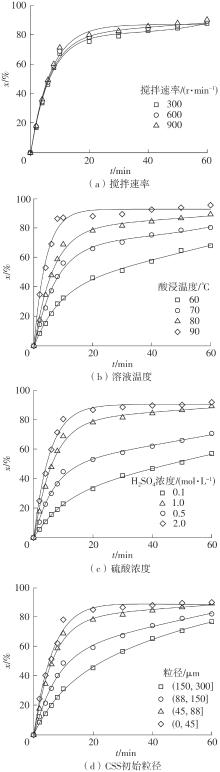
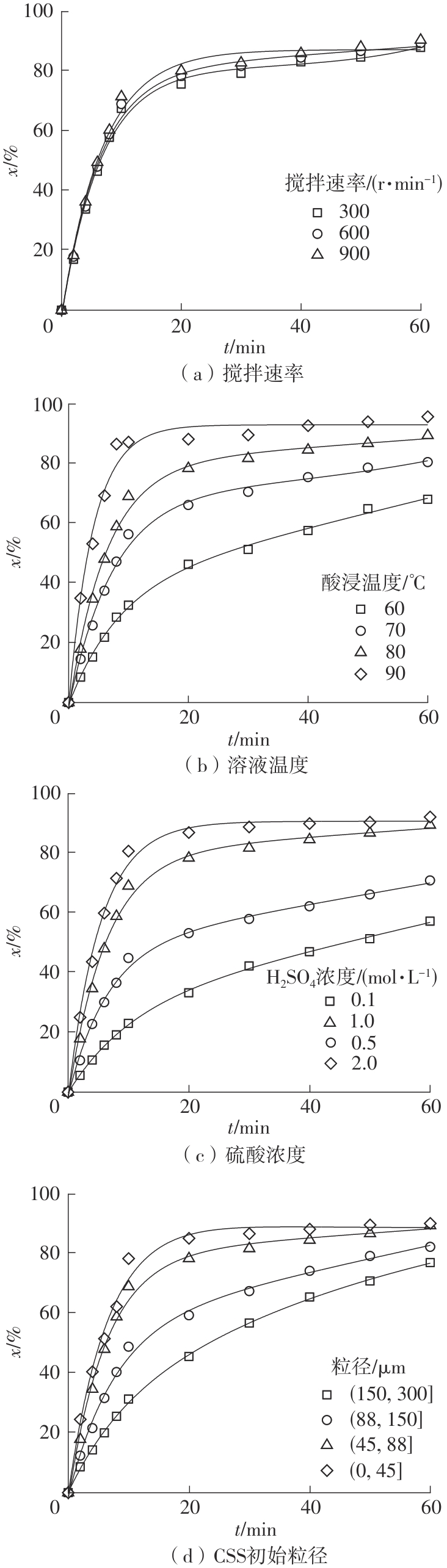
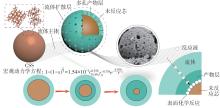
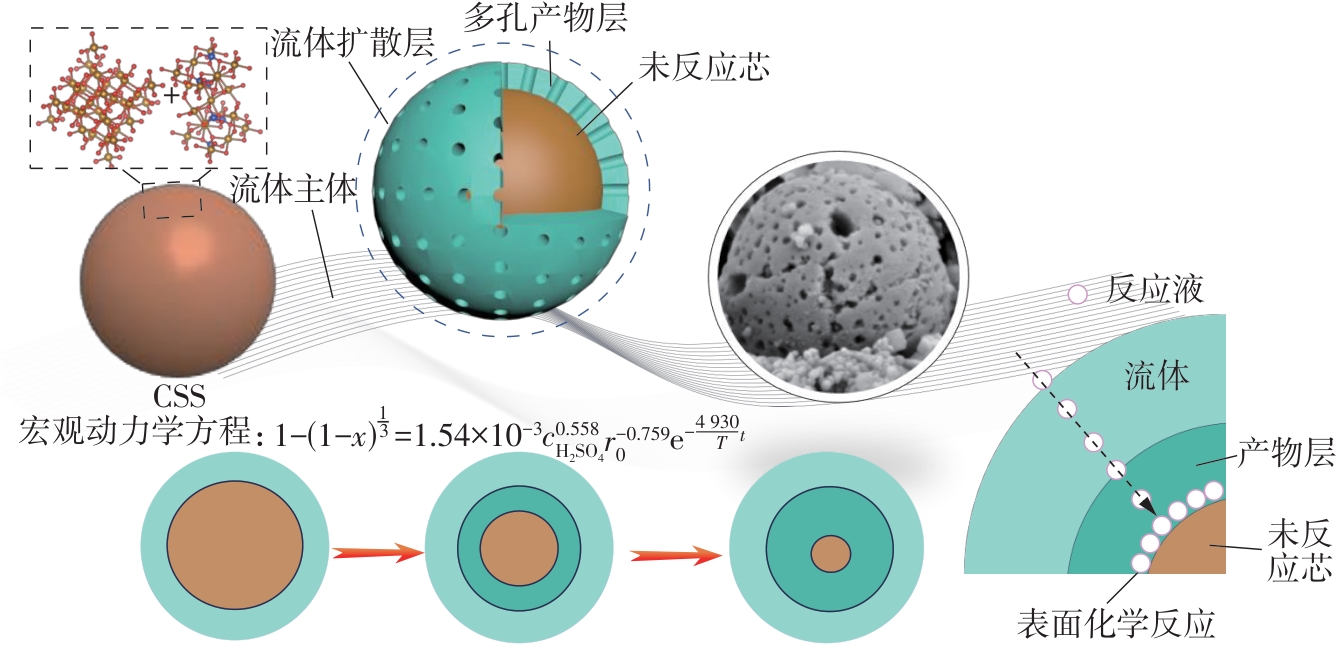
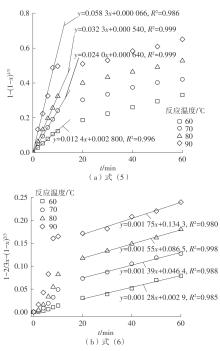
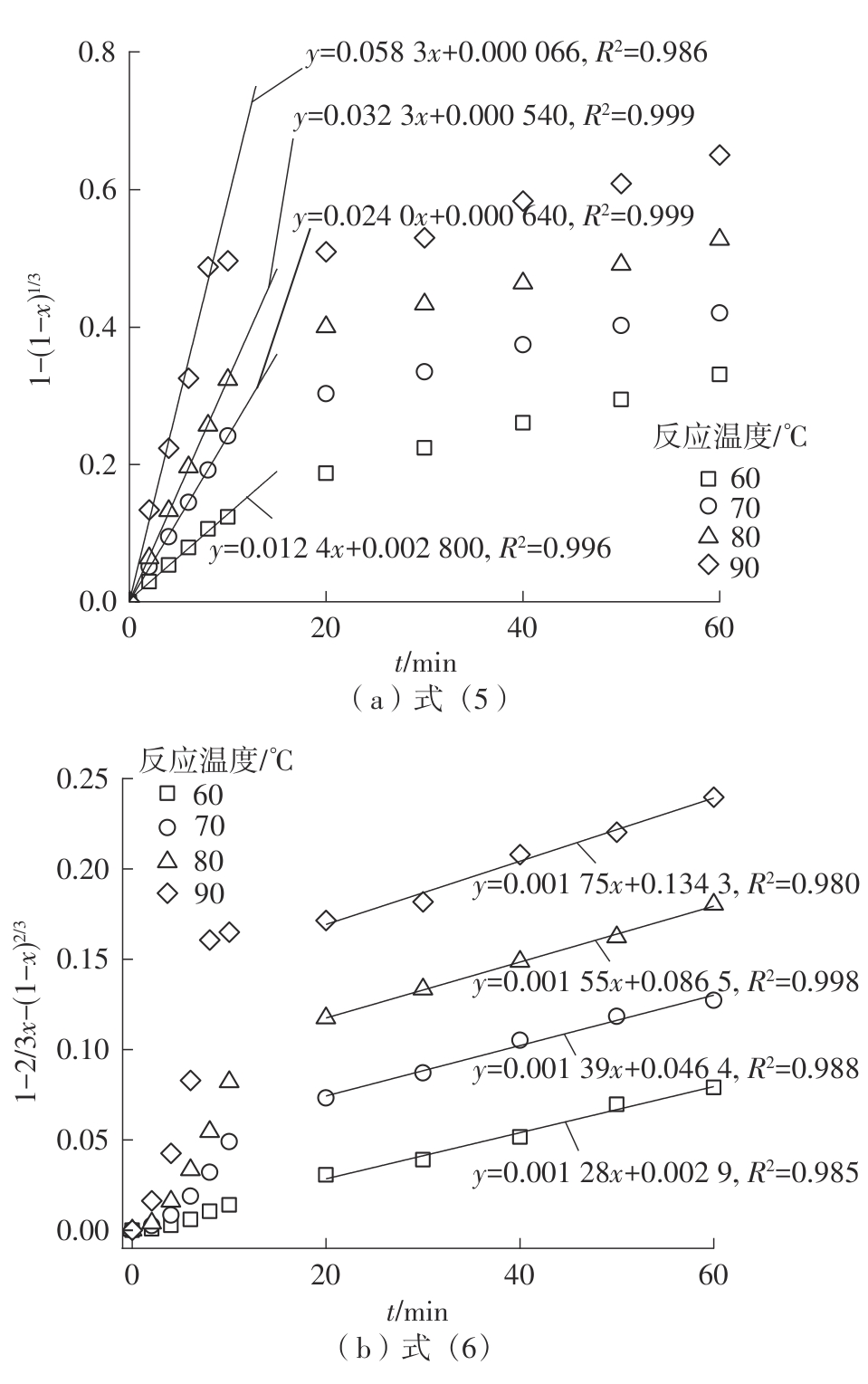
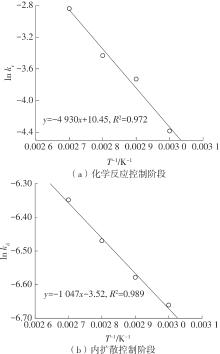
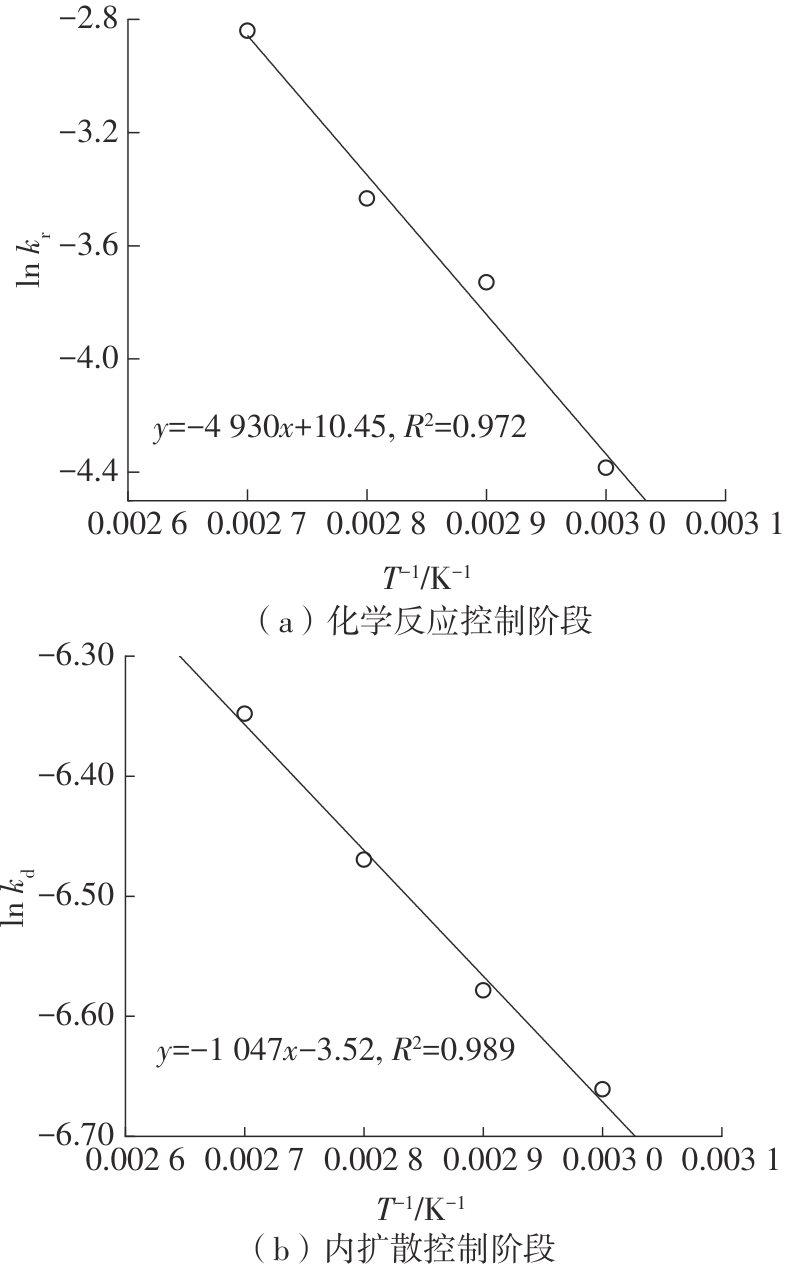
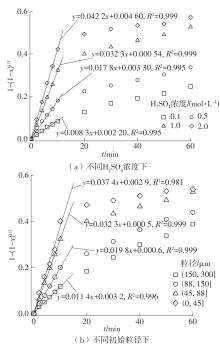
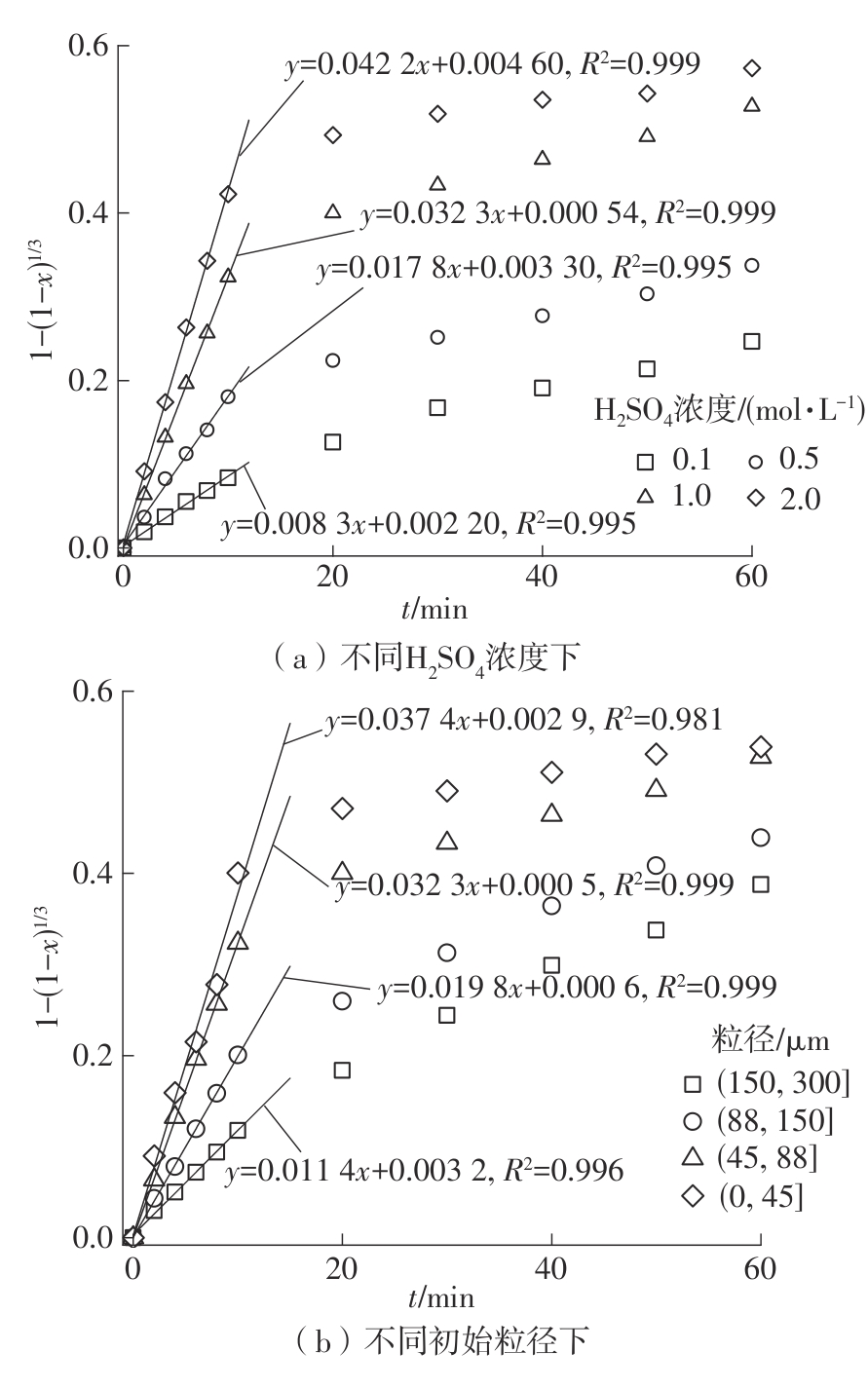
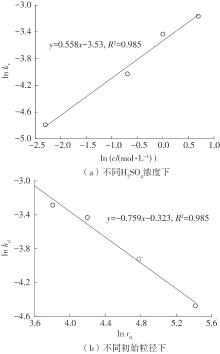
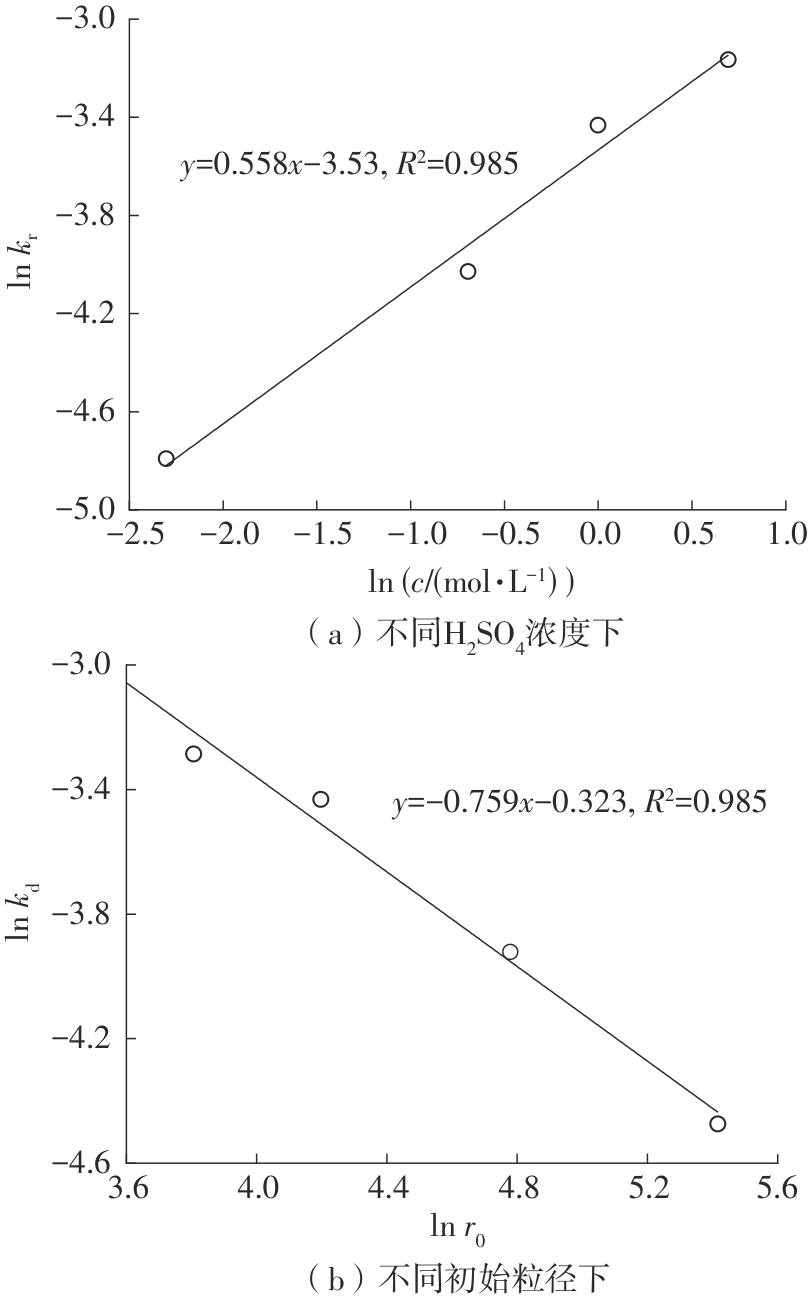
| 1 |
GUO H, WANG Z, AN D,et al .Collaborative design of cement-based composites incorporated with cooper slag in considerations of engineering properties and microwave-absorbing characters[J].Journal of Cleaner Production,2021,283:124614/1-14.
|
| 2 |
ZUO Z, YU Q, LUO S,et al .Effects of CaO on two-step reduction characteristics of copper slag using biochar as reducer:thermodynamic and kinetics[J].Energy Fuels,2020,34(1):491-500.
|
| 3 |
GUO Z, PAN J, ZHU D,et al .Green and efficient utilization of waste ferric-oxide desulfurizer to clean waste copper slag by the smelting reduction-sulfurizing process [J].Journal of Cleaner Production,2018,199:891-899.
|
| 4 |
殷素红,管海宇,胡捷,等 .碱激发粉煤灰-矿渣灌浆材料的流变性与流动性[J].华南理工大学学报(自然科学版),2019,47(8):120-128,135.
|
|
YIN Suhong, GUAN Haiyu, HU Jie,et al . Rheological properties and fluidity of alkali-activated fly ash-slag grouting material[J]. Journal of South China University of Technology(Natural Science Edition),2019,47(8):120-128,135.
|
| 5 |
YANG Z, QIAN J, YU A,et al .Singlet oxygen mediated iron-based Fenton-like catalysis under nanoconfinement[J].Proceedings of the National Academy of Sciences of the United States of America,2019,116(14):6659-6664.
|
| 6 |
WANG G, ZHOU A, XU Q . α-Ferrous oxalate with different micro scale:synthesis and catalytic degradation effect to rhodamine B[J].Solid State Sciences,2019,91:54-60.
|
| 7 |
WANG J, BAI Z .Fe-based catalysts for heterogeneous catalytic ozonation of emerging contaminants in water and wastewater[J].Chemical Engineering Journal,2017,312:79-98.
|
| 8 |
CLARIZIA L, RUSSO D, DI SOMMA I,et al .Homogeneous photo-Fenton processes at near neutral pH:a review[J].Applied Catalysis B:Environmental,2017,209:358-371.
|
| 9 |
GAI L, JIANG H, CUI D,et al .Room temperature blue-green photoluminescence of MCM-41,MCM-48 and SBA-15 mesoporous silicas in different conditions[J].Microporous and Mesoporous Materials,2009,120(3):410-413.
|
| 10 |
STÖBER W, FINK A, BOHN E .Controlled growth of monodisperse silica spheres in the micron size range[J]. Journal of Colloid Interface Sciences,1968,26(1):62-69.
|
| 11 |
WANG P, WANG X, YU S,et al .Silica coated Fe3O4 magnetic nanospheres for high removal of organic pollutants from wastewater[J].Chemical Engineering Journal,2016,306:280-288.
|
| 12 |
TEIMURI-MOFRAD R, TAHMASEBI S, PAYAMI E .Fe3O4@SiO2@Im-bisethylFc [HC2O4] as a novel recyclable heterogeneous nanocatalyst for synthesis of bis-coumarin derivatives[J].Applied Organometallic Chemistry,2019,33(6):4773/1-16.
|
| 13 |
HUANG X, WU S, KE X,et al .Phosphonated pillar[5]arene-valved mesoporous silica drug delivery systems[J]. ACS Applied Materials and Interfaces,2017,9(23):19638-19645.
|
| 14 |
LIN F-C, ZINK J I .Probing the local nanoscale heating mechanism of a magnetic core in mesoporous silica drug-delivery nanoparticles using fluorescence depolarization[J].Journal of the American Chemical Society,2020,142(11):5212-5220.
|
| 15 |
ZHANG T, ZHANG Q, GE J,et al .A self-templated route to hollow silica microspheres[J].Journal of Physical Chemistry C,2009,113(8):3168-3175.
|
| 16 |
WANG L, SU Q,IAN H,et al .Leaching behavior and occurrence of metal elements in copper slag:the key to recycling metals in copper slag[J].Journal of Hazardous Materials Advances,2023,12:100374/1-9.
|
| 17 |
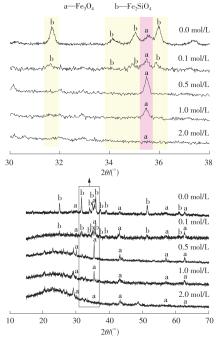
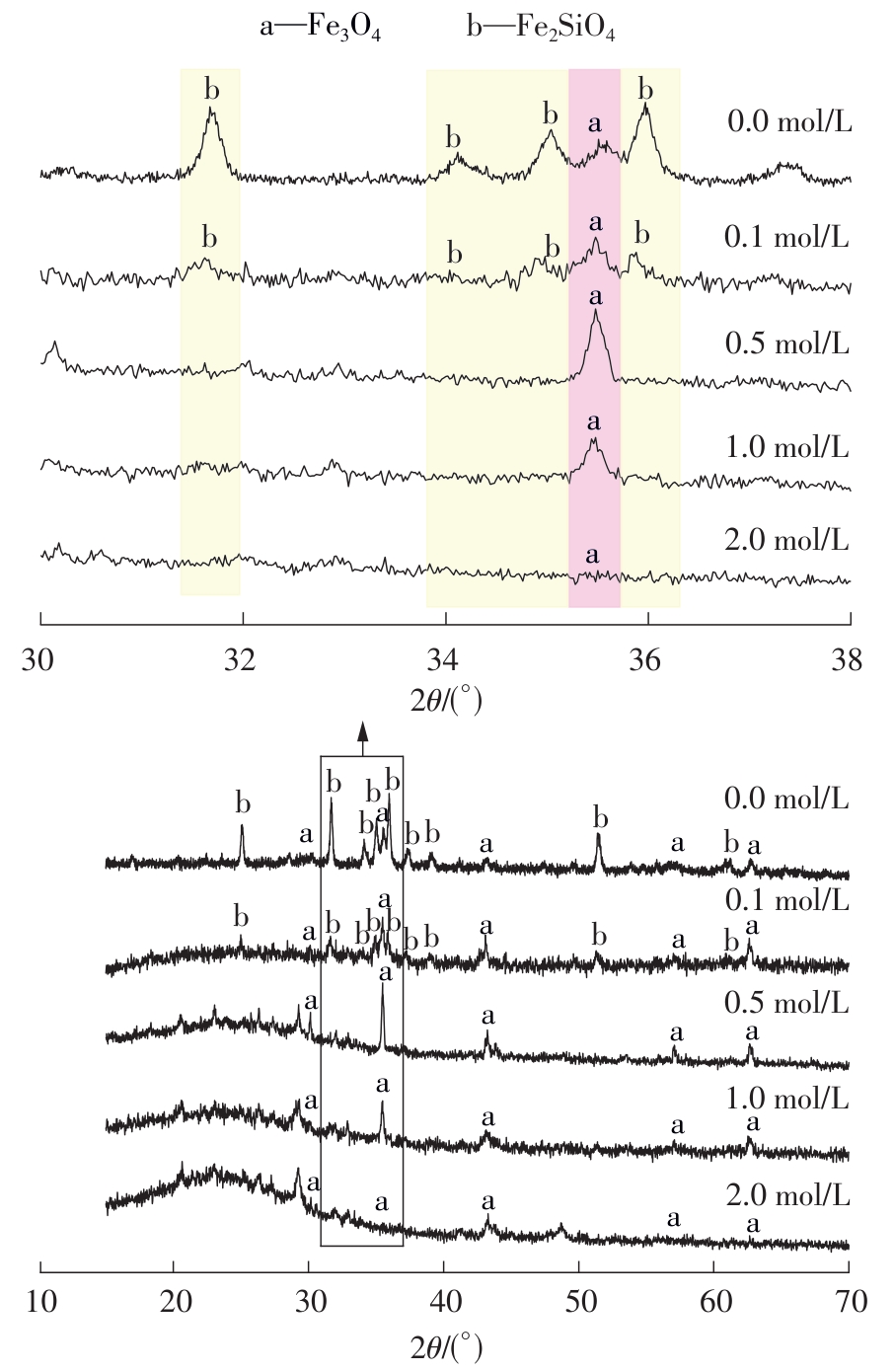
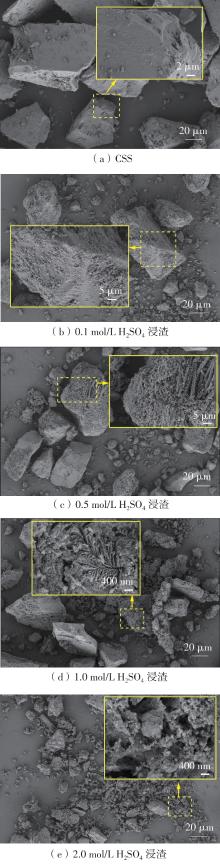
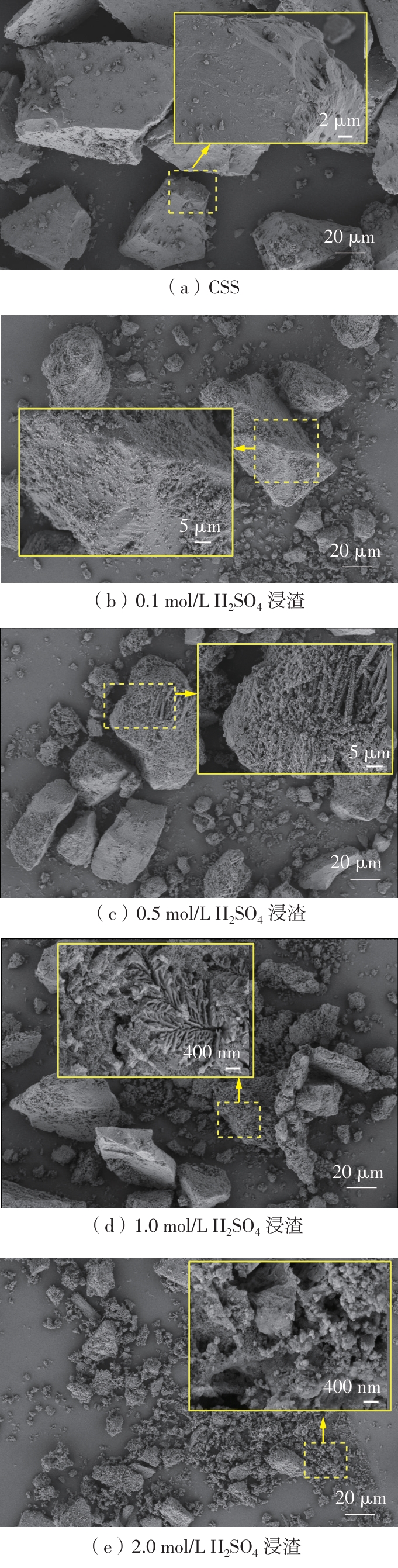
ZHANG S, ZHU N, MAO F,et al .A novel strategy for harmlessness and reduction of copper smelting slags by alkali disaggregation of fayalite (Fe2SiO4) coupling with acid leaching[J].Journal of Hazardous Materials,2021,402:123791/1-9.
|
| 18 |
SHI G, LIAO Y, SU B,et al .Kinetics of copper extraction from copper smelting slag by pressure oxidative leaching with sulfuric acid[J].Separation and Purification Technology,2020,241:116699/1-10.
|
| 19 |
LI L, WU G D, TIAN F G .Immobilization of fluorides from spent carbon cathode in a copper smelting slag[J].Journal of Mining and Metallurgy,Section B:Metallurgy,2022,58(1):129-139.
|
| 20 |
LUO Y, ZHOU X, LUO Z,et al .A novel iron phosphate cement derived from copper smelting slag and its early age hydration mechanism[J].Cement and Concrete Composites,2022,133:104653/1-13.
|
| 21 |
WOOD C E, QAFOKU O, LORING J S,et al .Role of Fe(Ⅱ) content in olivine carbonation in wet supercritical CO2 [J].Environmental Science & Technology Letters,2019,6(10):592-599.
|
| 22 |
TAO L, WANG L, YANG K,et al .Leaching of iron from copper tailings by sulfuric acid:behavior,kinetics and mechanism[J].RSC Advances,2021,11(10):5741-5752.
|
| 23 |
ALKAN M, DOǦN M, NAMLI H .Dissolution kinetics and mechanism of ulexite in oxalic acid solutions [J].Industrial & Engineering Chemistry Research,2004,43(7):1591-1598.
|
| 24 |
CAI X, TIAN L, CHEN M,et al .Construction of a C-decorated and Cu-doped (Fe,Cu)S/CuFe2O4 solid solution for photo-Fenton degradation of hydrophobic organic contaminant:enhanced electron transfer and adsorption capacity[J].Chemosphere,2022,296:134005/1-13.
|
| 25 |
温婧,姜涛,余唐霞,等 .钒铬渣锰盐焙烧酸浸过程中钒、铬的分离行为[J].中国有色金属学报,2021,31(4):977-983.
|
|
WEN Jing, JIANG Tao, YU Tang-xia,et al .Separation of vanadium and chromium from vanadium-chromium slag by manganese salt roasting[J].The Chinese Journal of Nonferrous Metals,2021,31(4):977-983.
|
| 26 |
ZHANG L, ZHU Y, YIN W,et al .Isothermal coal-based reduction kinetics of fayalite in copper slag[J].ACS Omega,2020,5(15):8605-8612.
|
| 27 |
MANJARREZ L, NIKVAR-HASSANI A, SHADNIA R,et al .Experimental study of geopolymer binder synthesized with copper mine tailings and low-calcium copper slag[J].Journal of Materials in Civil Engineering,2019,31(8):04019156/1-14.
|
| 28 |
WANG W-W, YAO J-L .Synthesis and magnetic property of silica/iron oxides nanorods[J].Materials Letters,2010,64(7):840-842.
|
| 29 |
XIAO W, LIU X, ZHAO Z .Kinetics of nickel leaching from low-nickel matte in sulfuric acid solution under atmospheric pressure[J].Hydrometallurgy,2020,194:105353/1-11.
|
| 30 |
RATH P C, PARAMGURU R K, JENA P K .Kinetics of dissolution of zinc sulphide in aqueous ferric chloride solution[J].Hydrometallurgy,1981,6(3/4):219-225.
|
| 31 |
高凌宇,杨喜云,吴玉楼,等 .蛇纹石酸浸渣碱溶脱硅反应动力学[J].中国有色金属学报,2023,33(8):2718-2728.
|
|
GAO Ling-yu, YANG Xi-yun, WU Yu-lou,et al .Kinetics of alkali-dissolving desilication reaction of serpentine acid leaching slag[J].The Chinese Journal of Nonferrous Metals,2023,33(8):2718-2728.
|
| 32 |
SILVA G DA .Kinetics and mechanism of the bacterial and ferric sulphate oxidation of galena[J].Hydrometallurgy,2004,75(1/2/3/4):99-110.
|
 ), ZHANG Hao3, ZHOU Xintao1(
), ZHANG Hao3, ZHOU Xintao1( ), LUO Zhongqiu1, CAI Xiunan1, GAO Zimeng1, SHI Jinyu1
), LUO Zhongqiu1, CAI Xiunan1, GAO Zimeng1, SHI Jinyu1