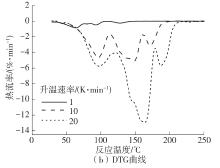
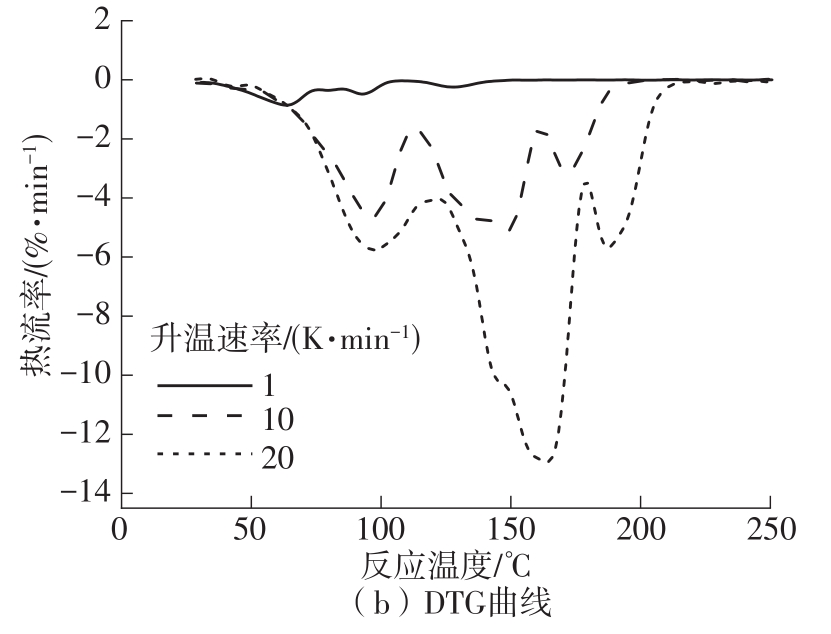
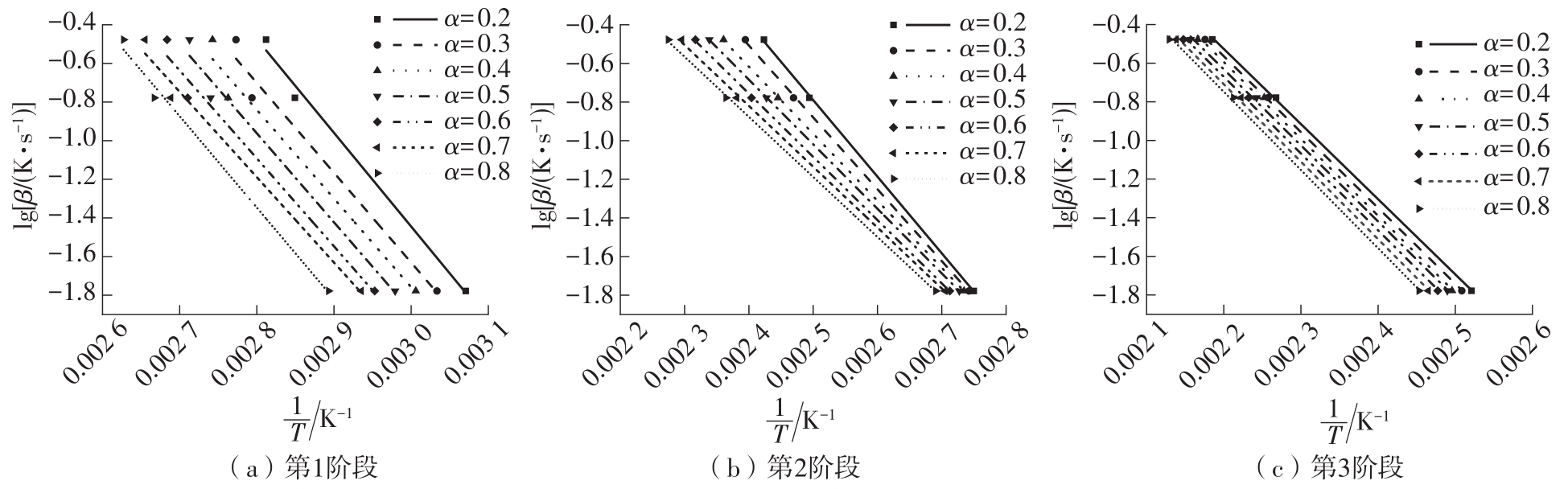
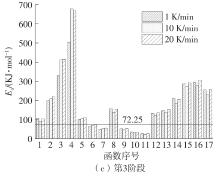
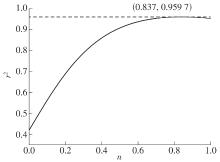
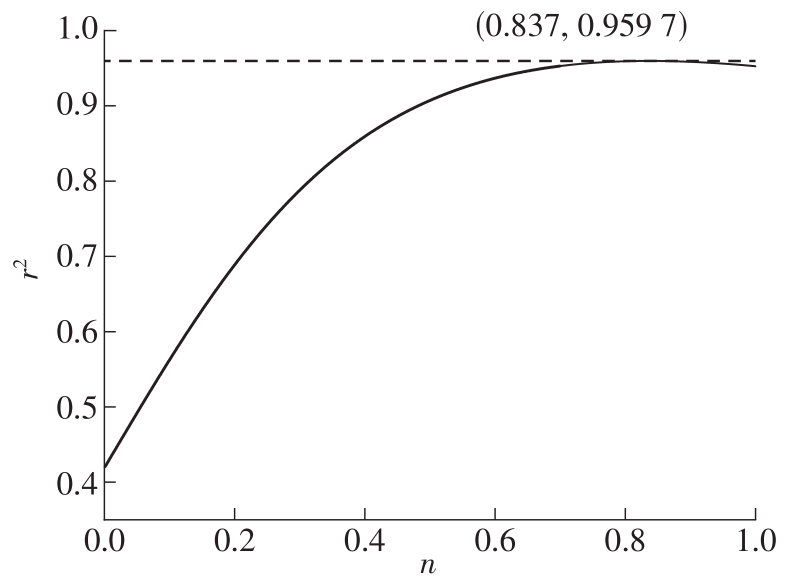
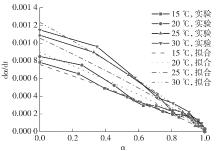
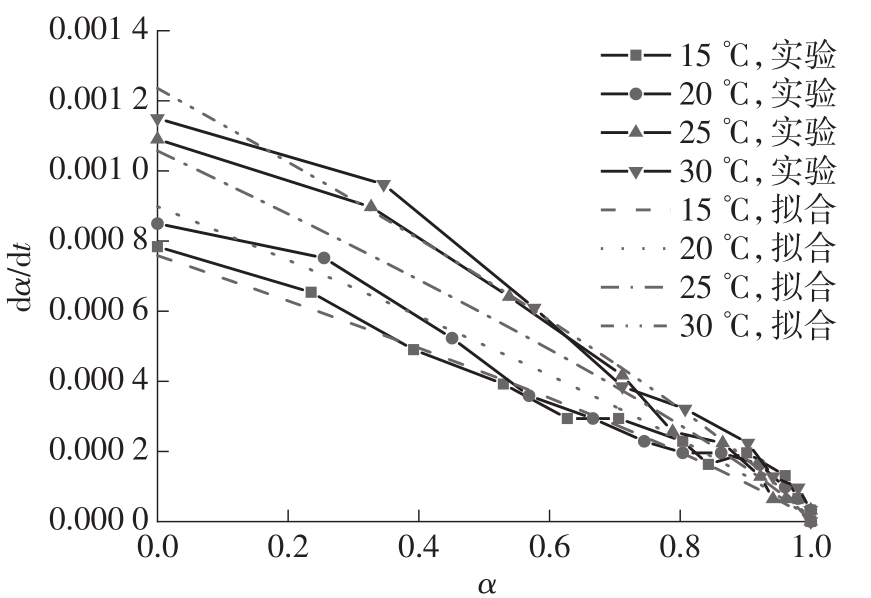
| 1 |
YU N, WANG R Z, WANG L W .Sorption thermal storage for solar energy[J].Progress in Energy and Combustion Science,2013,39(5):489-514.
|
| 2 |
AYDIN D, CASEY S P, RIFFAT S .The latest advancements on thermochemical heat storage systems[J].Renewable & Sustainable Energy Reviews,2015,41:356-367.
|
| 3 |
KERSKES H, METTE B, BERTSCH F,et al .Development of a thermo-chemical energy storage for solar thermal applications[C]∥ Proceedings of the ISES Solar World Congress 2011.Germany-Stuttgart:International Solar Energy Society,2011:4679-4685.
|
| 4 |
KOUSKSOU T, BRUEL P, JAMIL A,et al .Energy storage:Applications and challenges[J].Solar Energy Materials and Solar Cells,2014,120:59-80.
|
| 5 |
ZHAO B C, WANG R Z .Perspectives for short-term thermal energy storage using salt hydrates for building heating[J].Energy,2019,189:116139/1-6.
|
| 6 |
李威,陈威,王丹丹 .基于水合盐热化学储能的技术研究与进展[J].制冷与空调,2017,17(8):14-21.
|
|
LI Wei, CHEN Wei, WANG Dandan .Research and development of thermochemical energy storage based on hydrated salt[J].Refrigeration and Air-Conditioning,2017,17(8):14-21.
|
| 7 |
郝茂森,刘洪芝,王婉桐,等 .水合盐热化学储热材料的研究进展[J].储能科学与技术,2020,9(3):791-796.
|
|
HAO Maosen, LIU Hongzhi, WANG Wantong,et al .Researsh progress of thermochemical heat storage materials of hydrated salts[J].Energy Storage Science and Technology,2020,9(3):791-796.
|
| 8 |
翁立奎,张叶龙,姜琳,等 .基于水合盐的热化学吸附储热技术研究进展[J].储能科学与技术,2020,9(6):1729-1736.
|
|
WENG Likui, ZHANG Yelong, JIANG Lin,et al .Research progress on thermochemical adsorption heart storage technology based on hydrate[J].Energy Storage Science and Technology,2020,9(6):1729-1736.
|
| 9 |
TRAUSEL F, JONG A, CUYPERS R .A review on the properties of salt hydrates for thermochemical storage[J].Energy Procedia,2014,48:447-452.
|
| 10 |
BALASUBRAMANIAN G, GHOMMEM M, HAJJ M R,et al .Modeling of thermochemical energy storage by salt hydrates[J].International Journal of Heat and Mass Transfer,2010,53(25/26):5700-5706.
|
| 11 |
CLARK R J, MEHRABADI A, FARID M .State of the art on salt hydrate thermochemical energy storage systems for use in building applications[J].Journal of Energy Storage,2020,27:101145/1-18.
|
| 12 |
张雪龄,雷旭东,王菲菲,等 .水合无机盐热化学储热材料及技术研究进展[J].化工新型材料,2021,49(8):6-11.
|
|
ZHANG Xueling, LEI Xudong, WANG Feifei,et al .Research progress on hydrated salt thermochemical heat storage material and technology[J].New Chemical Materials,2021,49(8):6-11.
|
| 13 |
N'TSOUKPOE K E, SCHMIDT T, RAMMELBERG H U,et al .A systematic multi-step screening of numerous salt hydrates for low temperature thermochemical energy storage[J].Applied Energy,2014,124:1-16.
|
| 14 |
陈红兵,周昌勇,杨培志,等 .LaCl3:Ce3+晶体生长用无水氯化镧的制备[J].人工晶体学报,2006,35(4):686-691.
|
|
CHEN Hong-bing, ZHOU Chang-yong, YANG Pei-zhi,et al .Preparation of anhydrous lanthanum chloride for LaCl3:Ce3+ crystalg growth[J].Journal of Synthetic Crystals,2006,35(4):686-691.
|
| 15 |
龚政,陈佩珩,郭志箴,等 .七水氯化镧脱水过程的离解压和热效应[J].中国稀土学报,1985,3(2):13-16.
|
|
GONG Zheng, CHEN Peiheng, GUO Zhizhen,et al .Determination of dissociation pressure and thermal effect for dehydration process of lanthanum chloride heptahydrate[J].Journal of the Chinese Rare Earth Society,1985,3(2):13-16.
|
| 16 |
胡荣租,史启桢 .热分析动力学[M].北京:科学出版社,2001:1-18,47-112.
|
| 17 |
陈梅倩,胡德豪,黄友旺 .基于热重分析法的生物质变温热解特性实验研究[J].华北电力大学学报(自然科学版),2019,46(6):99-104.
|
|
CHEN Meiqian, HU Dehao, HUANG Youwang .Experimental study on pyrolysis characteristics of biomass based on thermogravimetric analysis[J].Journal of North China Electric Power University(Natural Science),2019,46(6):99-104.
|
| 18 |
CLARK R J, FARID M .Hydration reaction kinetics of SrCl2 and SrCl2-cement composite material for thermochemical energy storage[J].Solar Energy Materials and Solar Cells,2021,231:111311/1-14.
|
| 19 |
FISHER R, DING Y, SCIACOVELLI A .Hydration kinetics of K2CO3,MgCl2 and vermiculite-based composites in view of low-temperature thermochemical energy storage[J].Journal of Energy Storage,2021,38:102561/1-18.
|
| 20 |
FEDUNIK-HOFMAN L, BAYON A, DONNE S W .Kinetics of solid-gas reactions and their application to carbonate looping systems[J].Energies,2019,12(2981):1-35.
|
| 21 |
LELE A F, KUZNIK F, RAMMELBERG H U,et al .Thermal decomposition kinetic of salt hydrates for heat storage systems[J].Applied Energy,2015,154:447-458.
|
| 22 |
LIAVITSKAYA T, VYAZOVKIN S .Delving into the kinetics of reversible thermal decomposition of solids measured on heating and cooling[J].Journal of Physical Chemistry C,2017,121(28):15392-15401.
|
| 23 |
GAEINI M, SHAIK S A, RINDT C .Characterization of potassium carbonate salt hydrate for thermochemical energy storage in buildings[J].Energy and Buildings,2019,196:178-193.
|
| 24 |
沈维道,蒋智敏,童钧耕 .工程热力学[M].北京:高等教育出版社,2001:341-346.
|