华南理工大学学报(自然科学版) ›› 2023, Vol. 51 ›› Issue (3): 83-90.doi: 10.12141/j.issn.1000-565X.220385
所属专题: 2023年化学化工
聚丙烯酸在水溶液中的离解行为
李文波 杜萧萧
- 华南理工大学 材料科学与工程学院,广东 广州 510640
Dissociation Behavior of Polyacrylic Acid in Aqueous Solution
LI Wenbo DU Xiaoxiao
- School of Materials Science & Engineering,South China University of Technology,Guangzhou 510640,Guangdong,China
摘要:
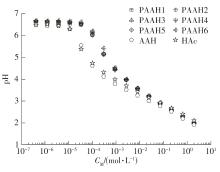
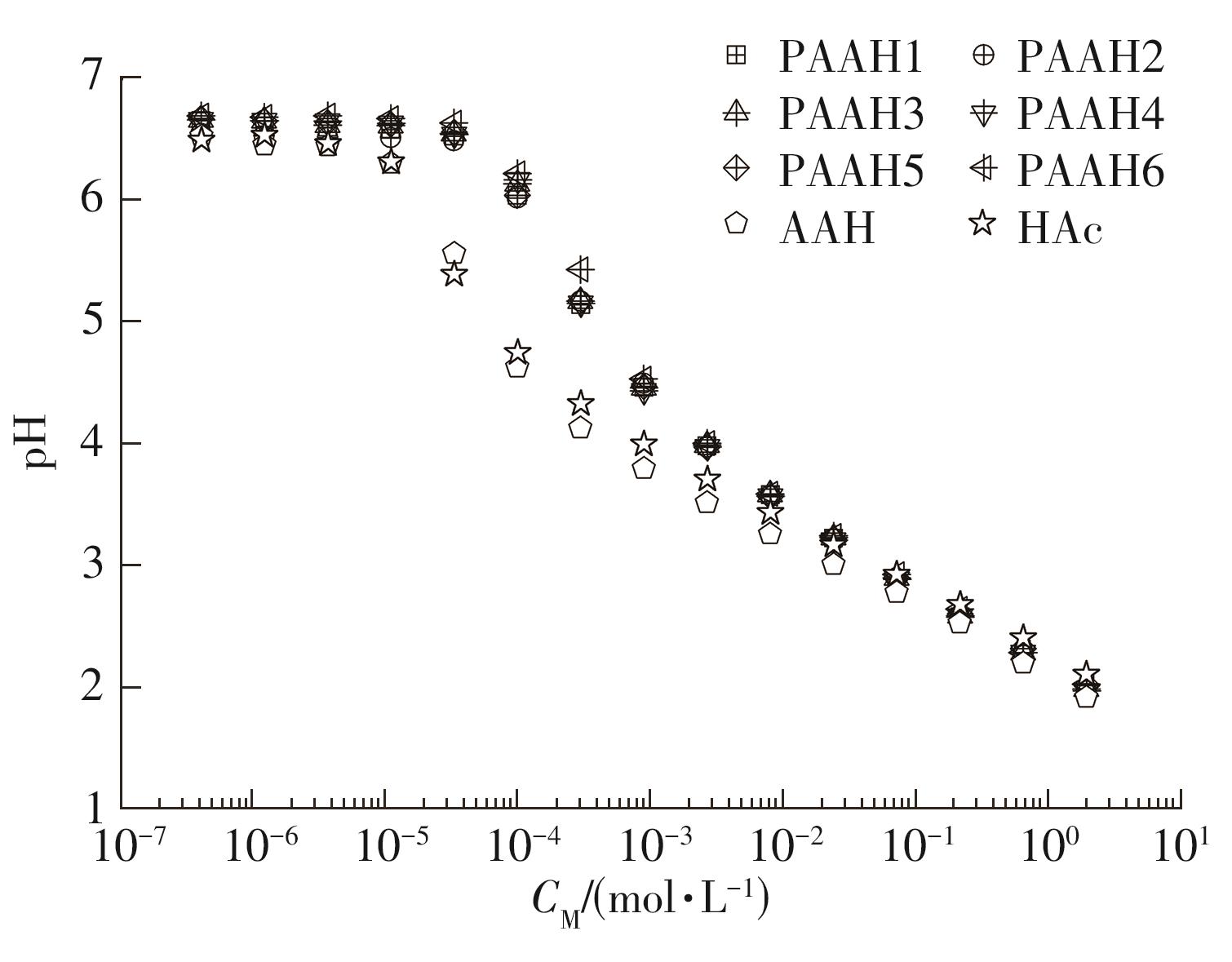
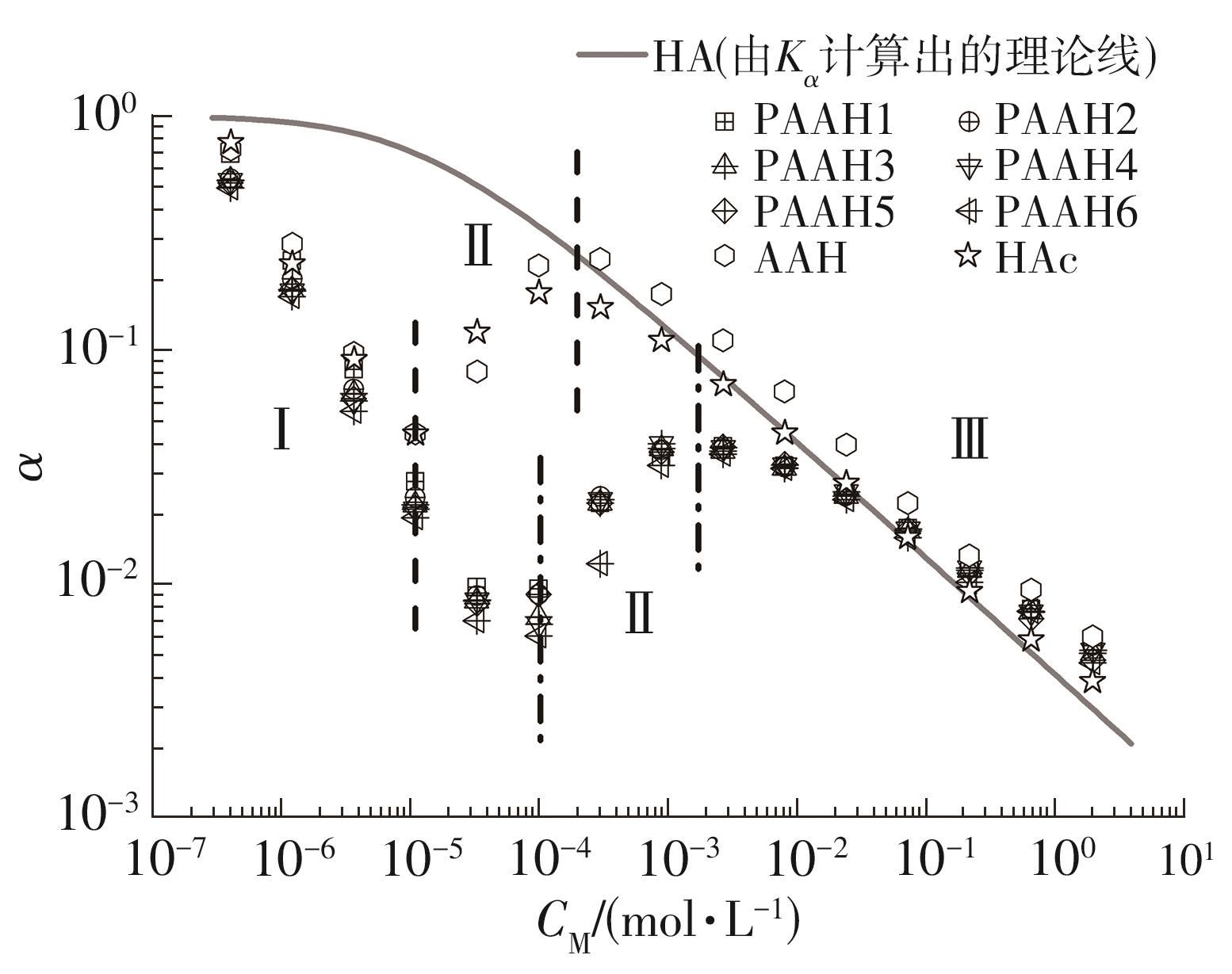
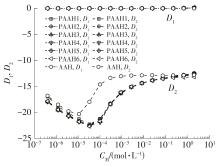
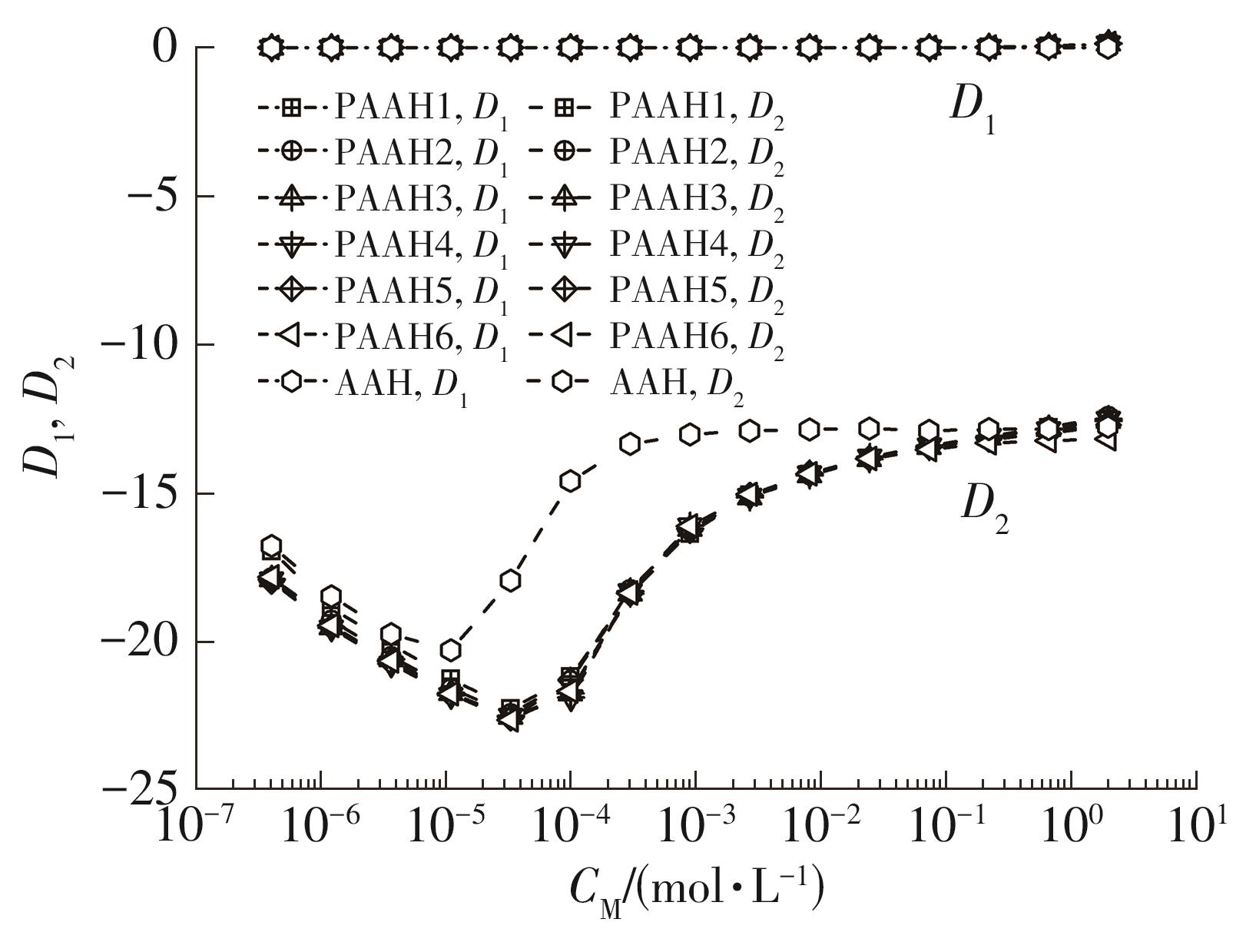
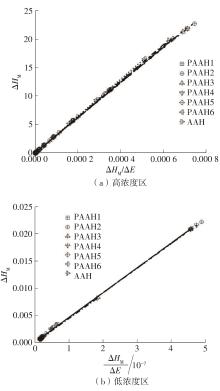
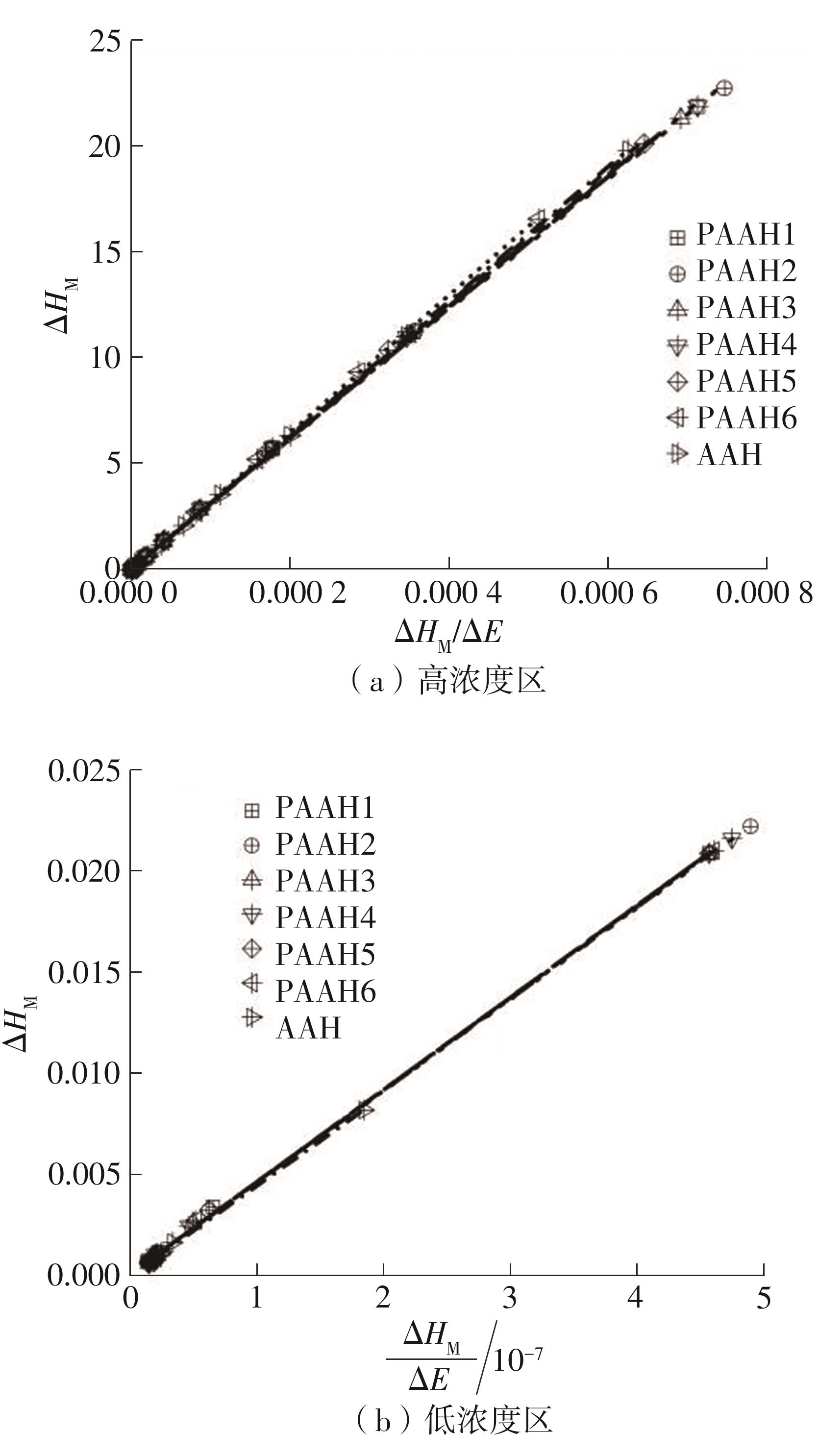
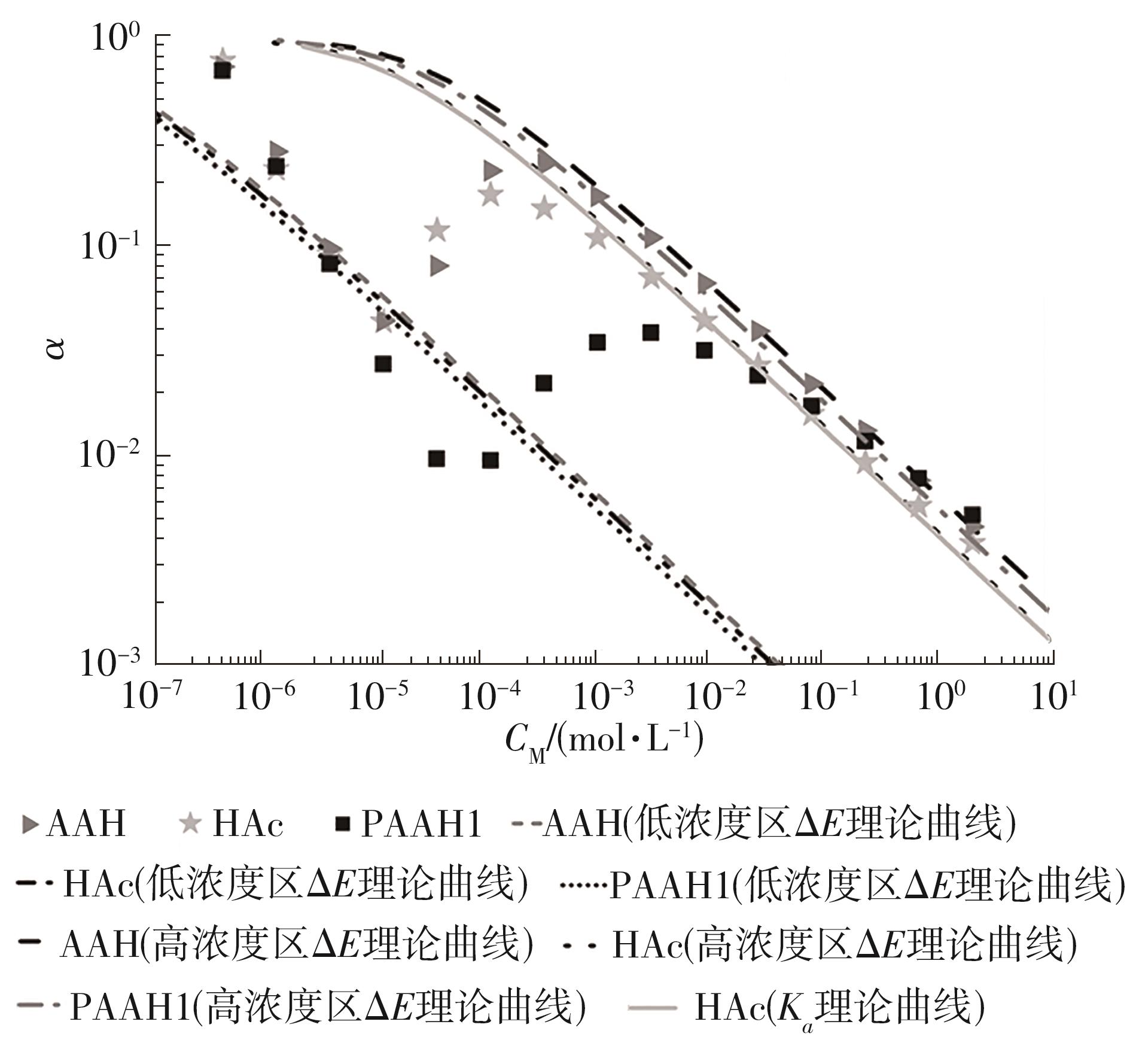
为了研究不同分子质量的聚丙烯酸(PAAH)在水溶液中的离解行为以及聚电解质(聚丙烯酸)与小分子弱电解质(丙烯酸(AAH)和醋酸(HAc))离解行为的差异。文中采用沉淀聚合法制备了聚合度在300~2 000范围内的聚丙烯酸,并使用pH计测量聚丙烯酸、丙烯酸和醋酸水溶液的pH值,以此来判定电解质在水溶液中的离解行为;然后从热力学角度和Flory-Huggins晶格模型推导出电解质在离解过程中的离解平衡方程。结果表明:聚丙烯酸在水溶液中的离解行为与聚合度基本无关。通过数学简化离解平衡方程得到传统平衡常数Kα 的计算公式,并赋予Kα 确切的物理意义:电解质水溶液在离解过程中离解水合效应相互作用能的相对强度参数。把数据代入离解平衡方程发现,聚电解质和小分子弱电解质均存在两种摩尔电离水合能ΔE,分别在低浓度区和高浓度区,这是丙烯酸、醋酸和聚丙烯酸在低浓度区都偏离了传统平衡常数Kα 理论线的原因。
中图分类号: