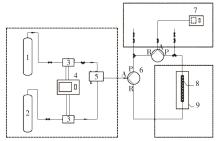
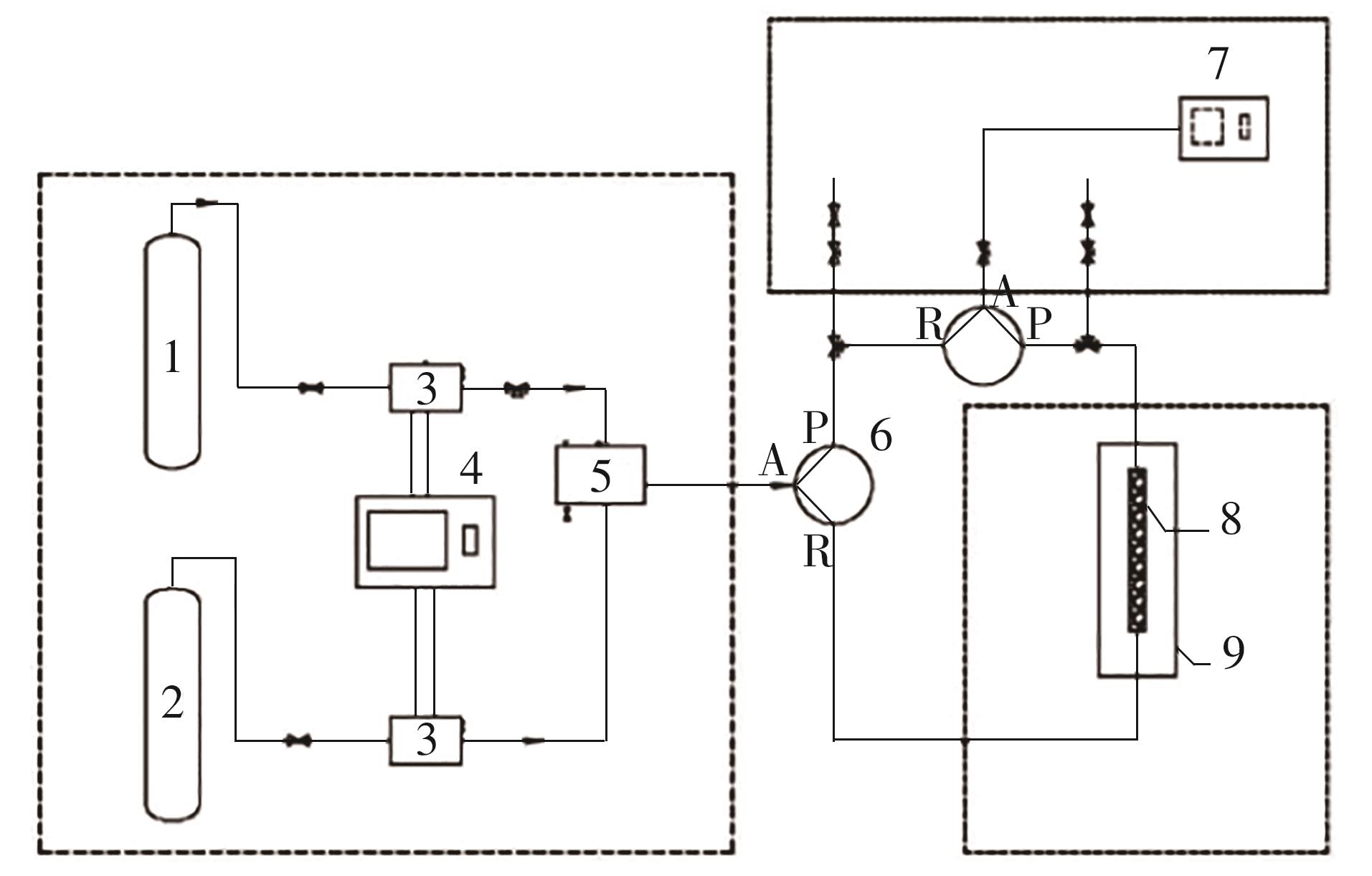
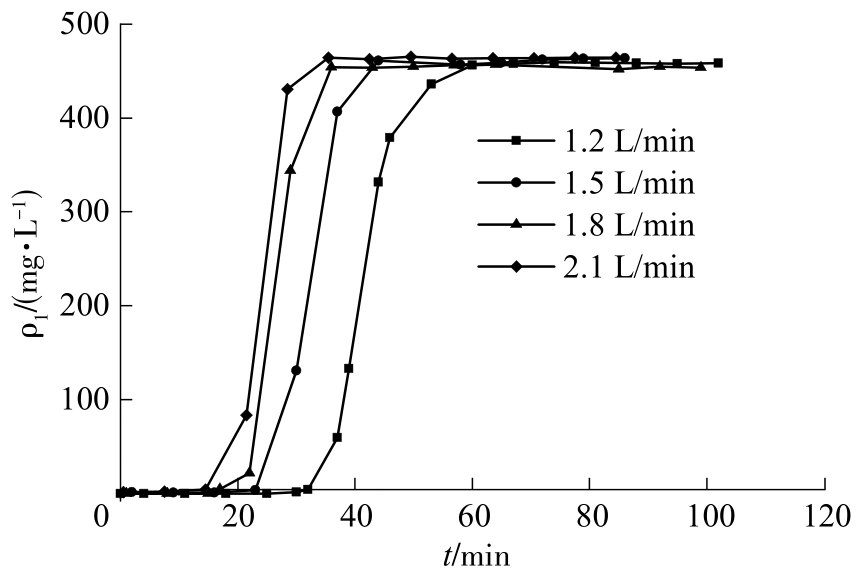
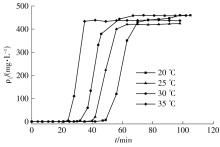
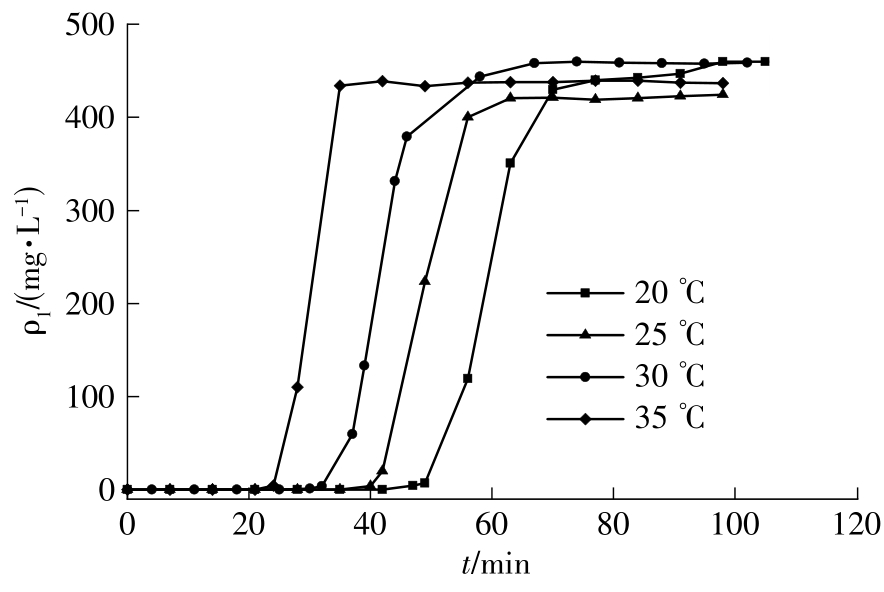
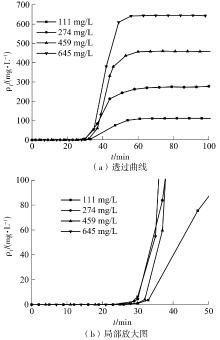
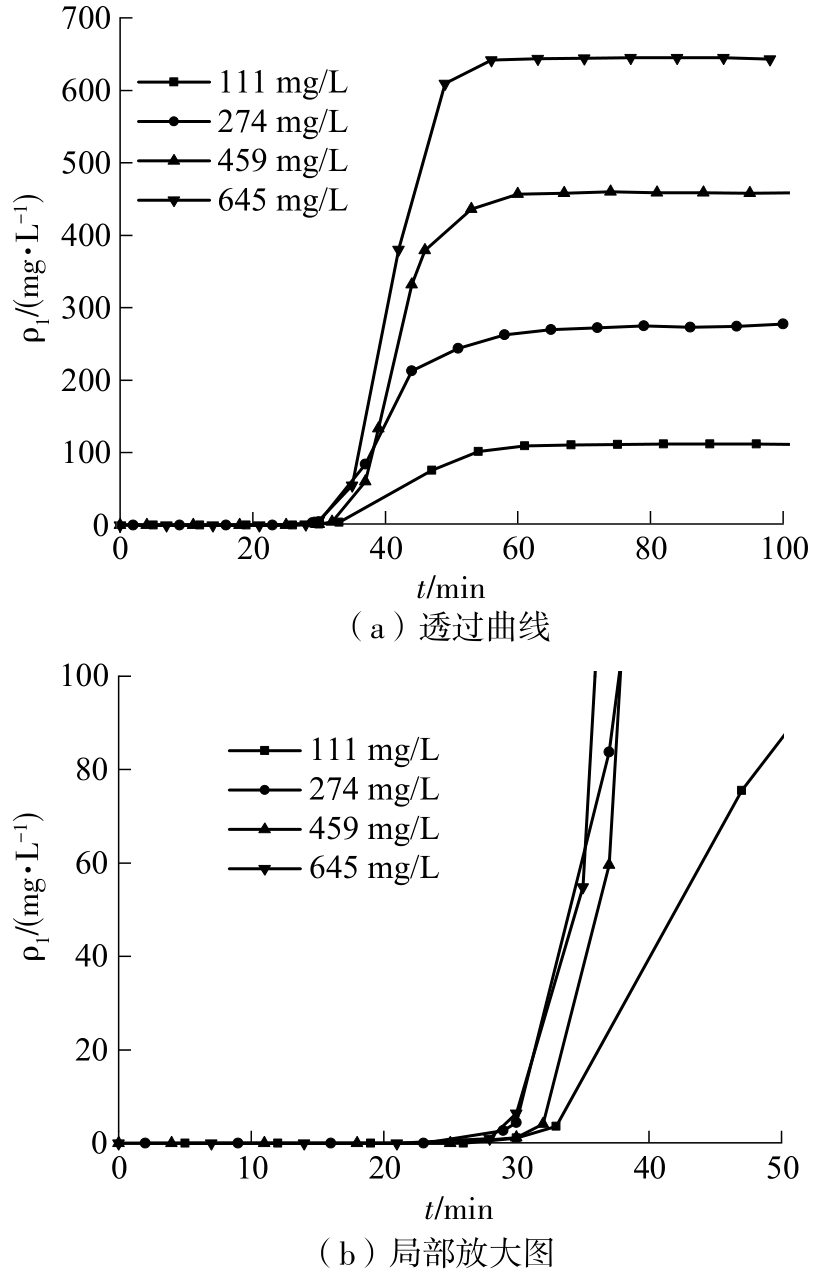
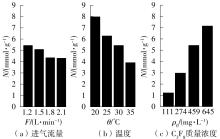
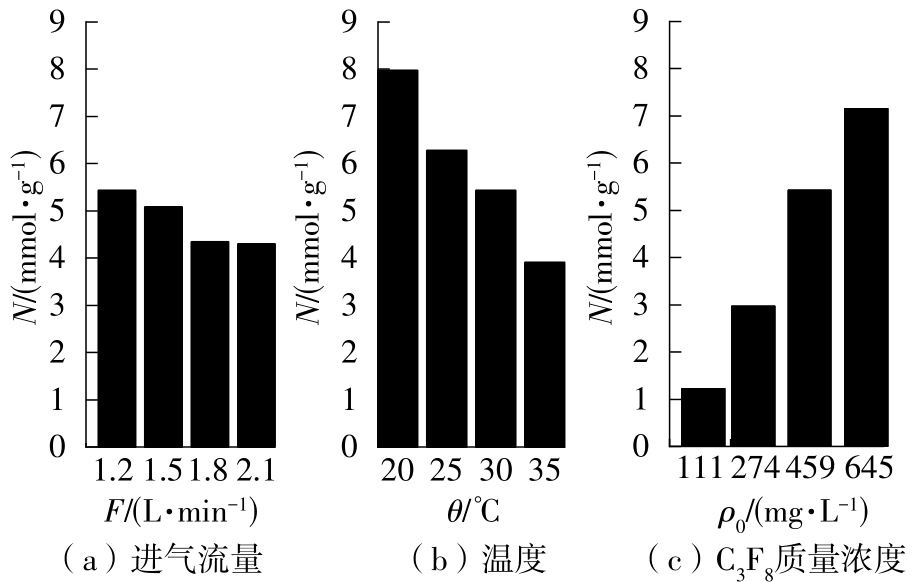
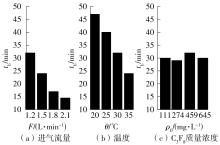
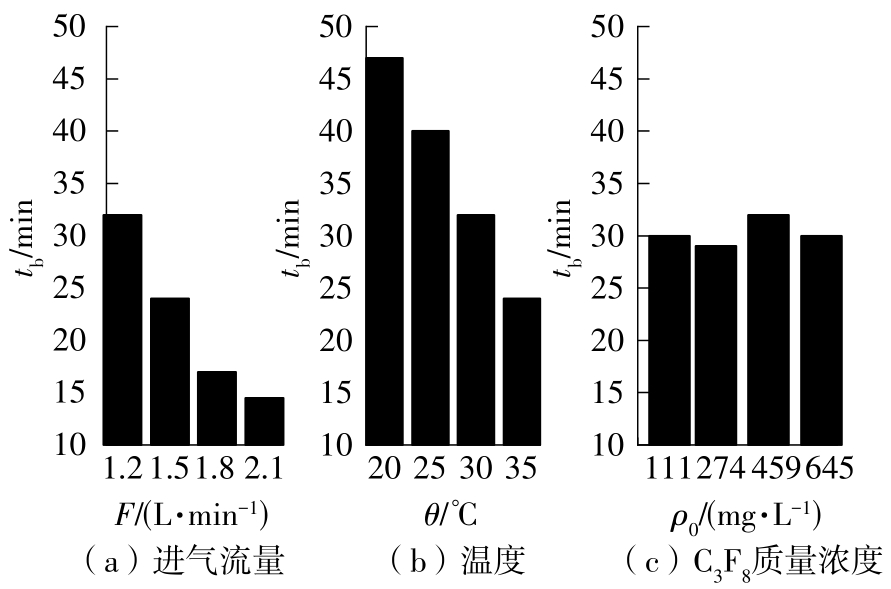
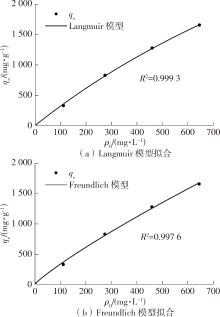
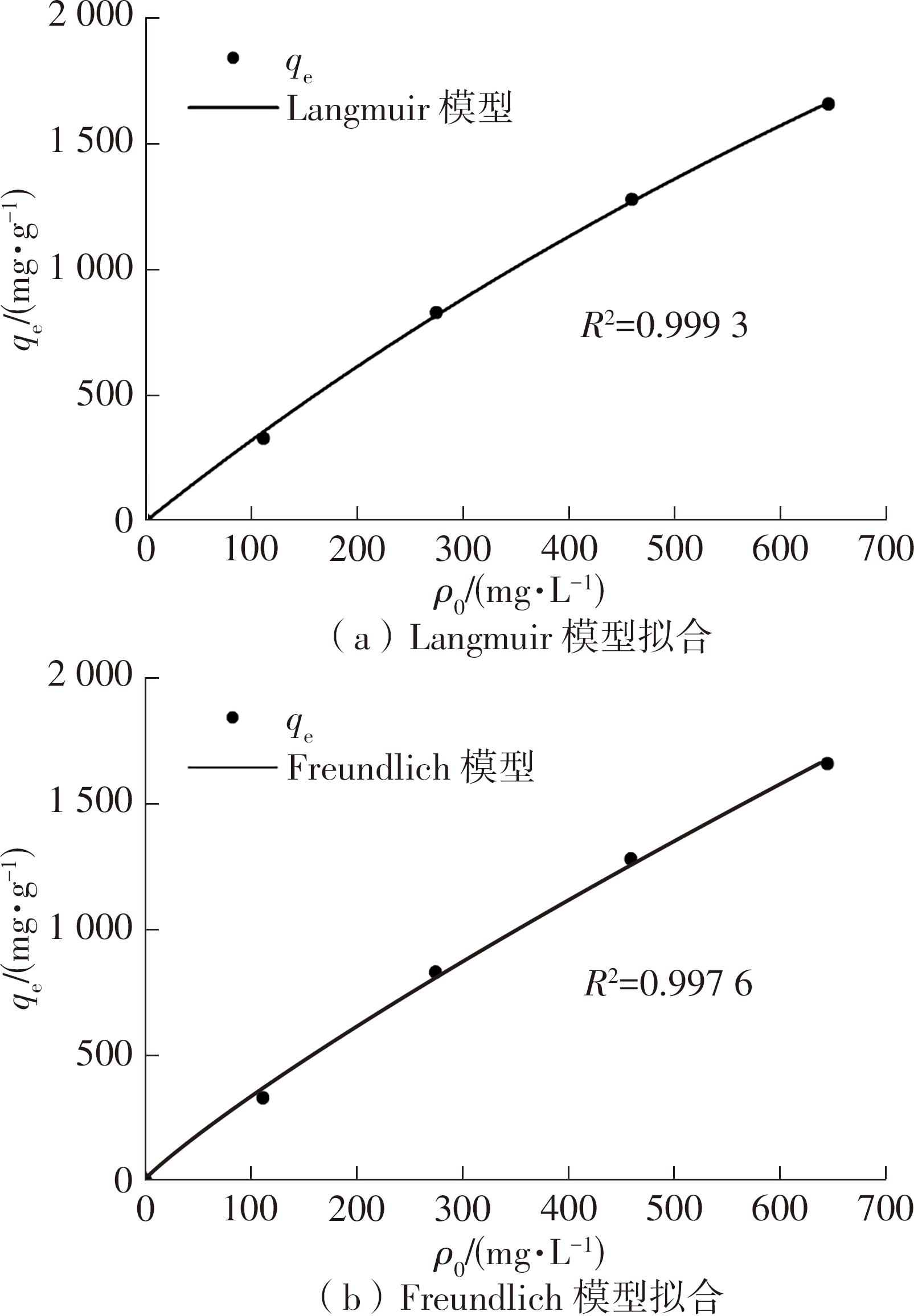
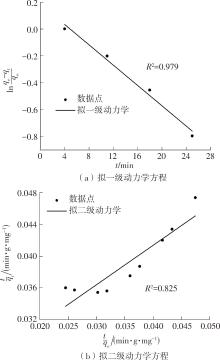
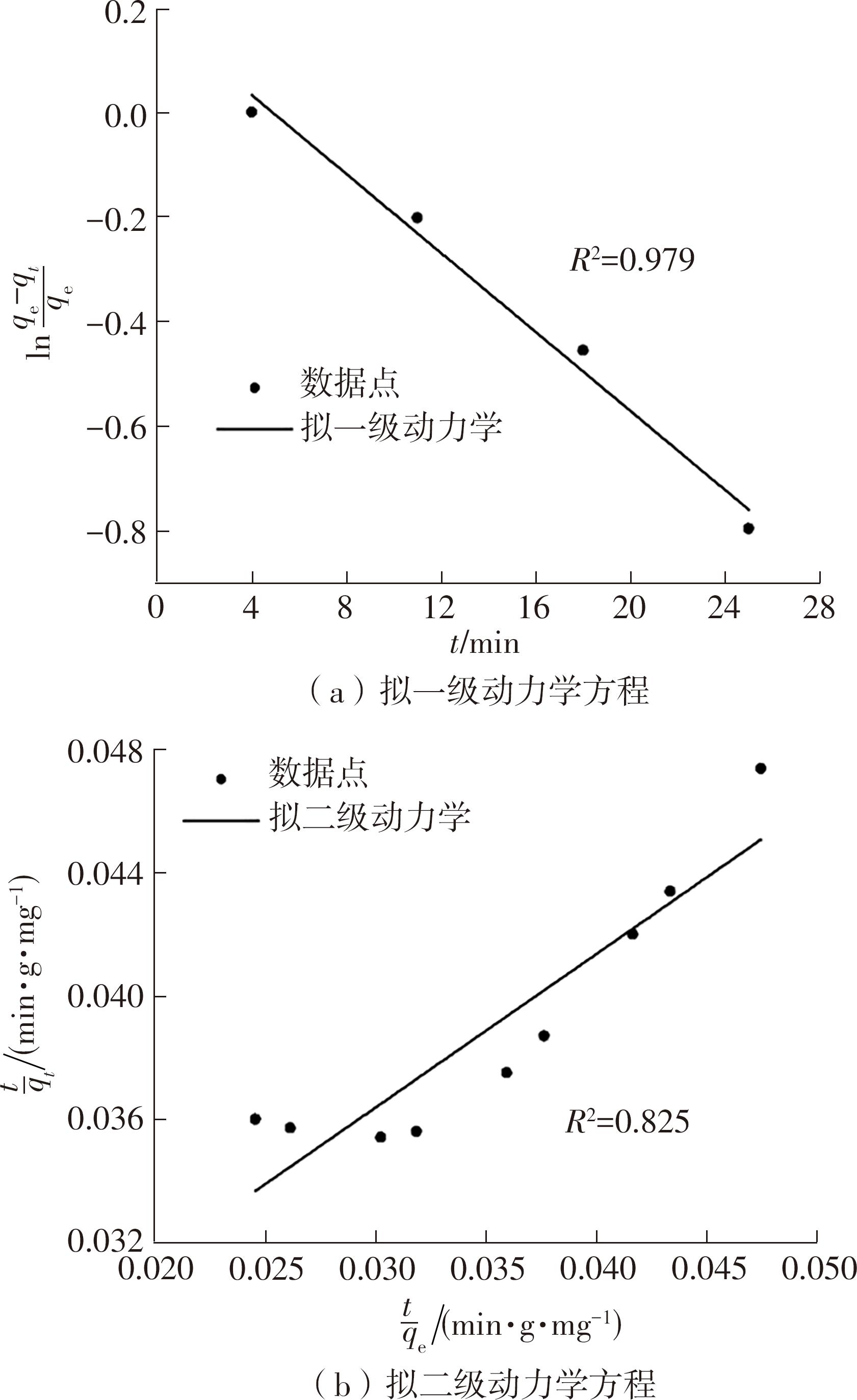
| 1 |
张亮 .电力设备中SF6混合绝缘气体的净化分离技术[J].黑龙江科学,2021,12(20):84-85.
|
|
ZHANG Liang .Purification separation technology of SF6 mixture insulating gas in the electrical equipment[J].Heilongjiang Science,2021,12(20):84-85.
|
| 2 |
廖恒易 .浅谈气体的分离和提纯[J].低温与特气,2015,33(6):5-7.
|
|
LIAO Hengyi .Gas separation and purification[J].Low Temperature and Specialty Gases,2015,33(6):5-7.
|
| 3 |
刘伟,苏镇西,祁炯,等 .电力设备中SF6混合绝缘气体净化分离技术的探究[J].高压电器,2016,52(12):227-231.
|
|
LIU Wei, SU Zhenxi, QI Jiong,et al .Investigation into the purification and separation technology for SF6 gas mixtures used in electrical apparatus[J].High Voltage Apparatus,2016,52(12):227-231.
|
| 4 |
LÜ D F, LIU Z W, XU F,et al .A Ni-based metal-organic framework with super-high C3H8 uptake for adsorptive separation of light alkanes[J].Separation and Purification Technology,2021,266:118198-1-10.
|
| 5 |
DU X D, PANG D D, CHENG Y G,et al .Adsorption of CH4,N2,CO2,and their mixture on montmorillonite with implications for enhanced hydrocarbon extraction by gas injection[J].Applied Clay Science,2021,210:106160-1-11.
|
| 6 |
WEBLEY P A .Adsorption technology for CO2 separation and capture:A perspective[J].Adsorption,2014,20(2/3):225-231.
|
| 7 |
김영훈,허려화, YOO Y D,et al .Analysis of CO2 adsorption characteristic of differently manufactured zeolite 13X[J].Journal of Energy & Climate Change,2008,3(1):34-40.
|
| 8 |
LAMIA N, WOLFF L, LEFLAIVE P,et al .Propane/propylene separation by simulated moving bed I.Adsorption of propane,propylene and isobutane in pellets of 13X zeolite[J].Separation Science and Technology,2007,42(12):2539-2566.
|
| 9 |
de OLIVEIRA L H, MENEGUIN J G, PEREIRA M V,et al .H2S adsorption on NaY zeolite[J].Microporous and Mesoporous Materials,2019,284:247-257.
|
| 10 |
胡苏阳,刘鑫博,唐建峰,等 .13X沸石分子筛对低浓度CO2动态吸附[J].化工进展,2022,41(1):153-160.
|
|
HU Suyang, LIU Xinbo, TANG Jianfeng,et al .Dynamic adsorption of low concentration CO2 over 13X zeolite[J].Chemical Industry and Engineering Progress,2022,41(1):153-160.
|
| 11 |
HONG T Q, WEI L, CUI K P,et al .Adsorption performance of volatile organic compounds on activated carbon fibers in a fixed bed column[J].Journal of Environmental Chemical Engineering,2021,9(6):106347-1-8.
|
| 12 |
GUO Z H, SUN Z N, ZHANG N,et al .Mean porosity variations in packed bed of monosized spheres with small tube-to-particle diameter ratios[J].Powder Technology,2019,354:842-853.
|
| 13 |
MCCABE W L S J C, HARRIOTT P .Unit operations of chemical engineering[M].7th ed.New York:McGraw-Hill College,2004:110-133.
|
| 14 |
唐进京,鲁军辉,李俊明,等 .CO2/H2O吸附分离特性研究[J].中国电机工程学报,2022,42(6):2216-2227.
|
|
TANG Jinjing, LU Junhui, LI Junming,et al .Study on adsorption characteristics of CO2/H2O[J].Proceedings of the CSEE,2022,42(6):2216-2227.
|
| 15 |
SHENDALMAN L H, MITCHELL J E .A study of heatless adsorption in the model system CO2 in He,I[J].Chemical Engineering Science,1972,27(7):1449-1458.
|
| 16 |
POURSAEIDESFAHANI A, ANDRES-GARCIA E, de LANGE M,et al .Prediction of adsorption isotherms from breakthrough curves[J].Microporous and Mesoporous Materials,2019,277:237-244.
|
| 17 |
CRESPO D, QI G, WANG Y,et al .Superior sorbent for natural gas desulfurization[J].Industrial & Engineering Chemistry Research,2008,47(4):1238-1244.
|
| 18 |
DA N E, SAYARI A .Modeling adsorption of copper on amine-functionalized SBA-15:Predicting breakthrough curves[J].Journal of Environmental Engineering,2013,139(1):95-103.
|
| 19 |
DOWNAROWICZ D, ALEKSANDRZAK T .Isobutanol vapor adsorption on activated carbons:Equilibrium and kinetic studies[J].Journal of Chemical and Engineering Data,2017,62(10):3518-3524.
|
| 20 |
HAMOUDI S, SAAD R, BELKACEMI K .Modeling breakthrough curves for adsorption of monobasic phosphate using ammonium-functionalized MCM-48[J].Separation Science and Technology,2013,48(14):2099-2107.
|
| 21 |
NG M, SCHORK J M, FABREGAS K R .The mass transfer zone in nitrogen PSA columns[J].Gas Separation & Purifications,1993,7(3):159-166.
|
| 22 |
ZHANG Y H, JIN F, SHEN Z T,et al .Adsorption of methyl tert-butyl ether (MTBE) onto ZSM-5 zeolite:Fixed-bed column tests,breakthrough curve modelling and regeneration[J].Chemosphere,2019,220:422-431.
|
| 23 |
CHERN J M, CHIEN Y W .Adsorption of nitrophenol onto activated carbon:Isotherms and breakthrough curves[J].Water Research,2002,36(3):647-655.
|
| 24 |
FOMKIN A A, NIKIFOROV Y V, SINITSYN V A,et al .Adsorption of perfluoropropane on the PAC microporous carbon adsorbent[J].Russian Chemical Bulletin,2002,51(12):2161-2164.
|
| 25 |
YANG C F O, KAM S H, LIU C H,et al .Assessment of removal efficiency of perfluorocompounds (PFCs) in a semiconductor fabrication plant by gas chromatography[J].Chemosphere,2009,76(9):1273-1277.
|
| 26 |
LIN B Y, CHANG M B, CHEN H L,et al .Removal of C3F8 via the combination of non-thermal plasma,adsorption and catalysis[J].Plasma Chemistry and Plasma Processing,2011,31(4):585-594.
|
| 27 |
KOLOKOLOV D I, ARZUMANOV S S, FREUDE D,et al .Mobility of stable π-complexes of ethylene with Ag+ cations in Ag/H-ZSM-5 zeolite:A 2H solid-state NMR study[J].The Journal of Physical Chemistry C,2016,120(9):4993-5000.
|
| 28 |
ZHANG X, CHEN S, BI H T .Application of wave propagation theory to adsorption breakthrough studies of toluene on activated carbon fiber beds[J].Carbon,2010,48(8):2317-2326.
|
| 29 |
YI H, YANG X, TANG X,et al .Removal of toluene from industrial gas over 13X zeolite supported catalysts by adsorption-plasma catalytic process[J].Journal of Chemical Technology and Biotechnology,2017,92(9):2276-2286.
|
| 30 |
CHEN S J, FU Y, HUANG Y X,et al .Experimental investigation of CO2 separation by adsorption methods in natural gas purification[J].Applied Energy,2016,179:329-337.
|
| 31 |
JAHROMI P E, FATEMI S, VATANI A,et al .Purification of helium from a cryogenic natural gas nitrogen rejection unit by pressure swing adsorption[J].Separation and Purification Technology,2018,193:91-102.
|
| 32 |
HAJILARI M, SHARIATI A, KHOSRAVI-NIKOU M .Mass transfer determination of ethanol adsorption on activated carbon:Kinetic adsorption modeling[J].Heat and Mass Transfer,2019,55(8):2165-2171.
|