

收稿日期: 2018-11-15
修回日期: 2018-12-28
网络出版日期: 2019-08-01
基金资助
国家自然科学基金资助项目(31471691,31771906)
Expression of Wheat Endoplasmic Reticulum Oxidoreductin 1 in Pichia pastoris
Received date: 2018-11-15
Revised date: 2018-12-28
Online published: 2019-08-01
Supported by
Supported by the National Natural Science Foundation of China(31471691,31771906)
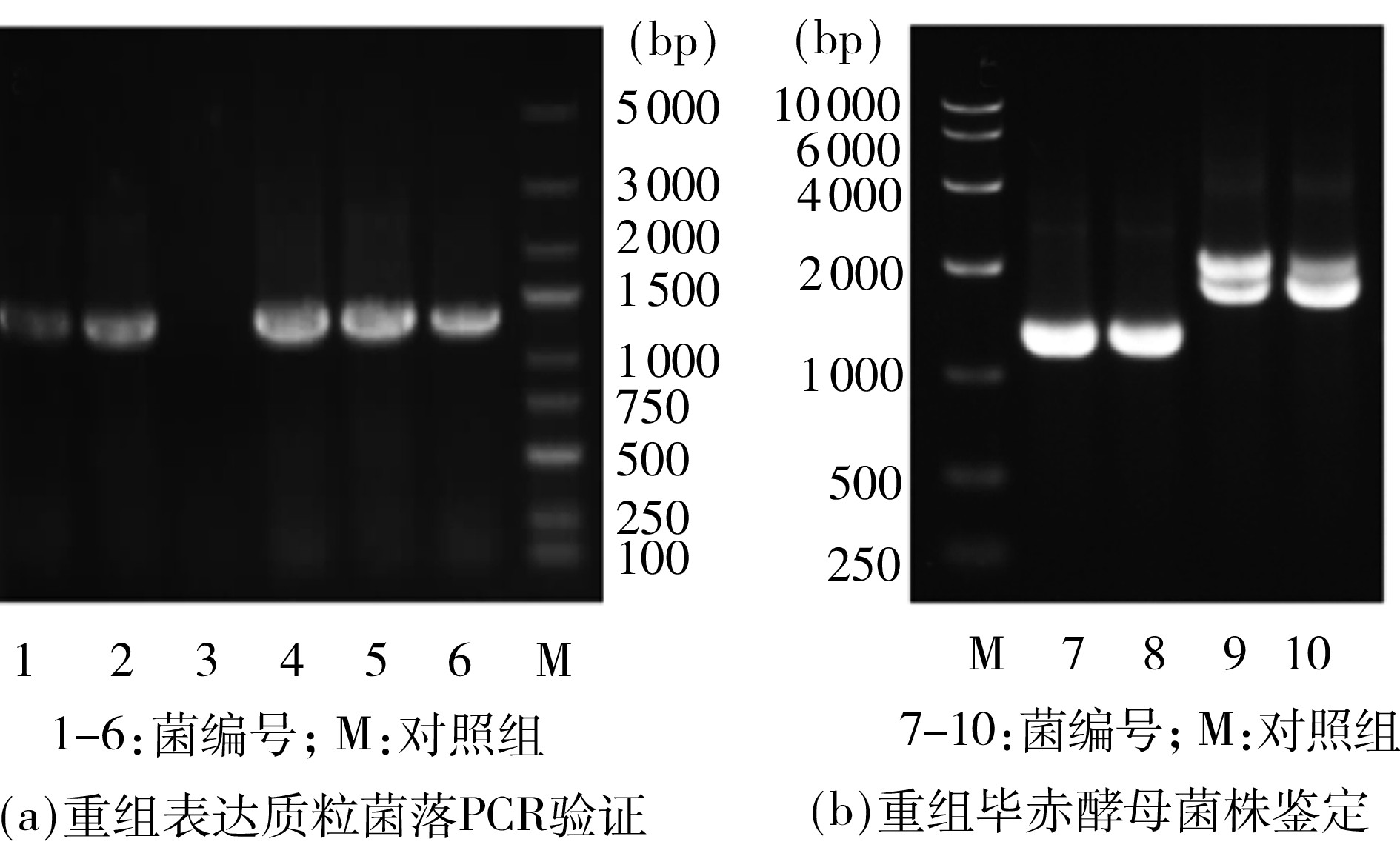
关键词: 小麦内质网氧化还原酶 1; 重组蛋白; 巯基氧化; 面粉粉质
胡松青 李旺 陈煜 侯轶 . 小麦内质网氧化还原酶在毕赤酵母中的表达[J]. 华南理工大学学报(自然科学版), 2019 , 47(8) : 57 -63,70 . DOI: 10.12141/j.issn.1000-565X.180567

/
| 〈 |
|
〉 |