

收稿日期: 2024-05-15
网络出版日期: 2024-12-24
基金资助
国家自然科学基金项目(52376108);广东省科技计划项目(2022A0505050004)
Effect of Ozone on Polycyclic Aromatic Hydrocarbon Formation in Combustion of Biodiesel Surrogate
Received date: 2024-05-15
Online published: 2024-12-24
Supported by
the National Natural Science Foundation of China(52376108);the Science and Technology Planning Project of Guangdong Province(2022A0505050004)
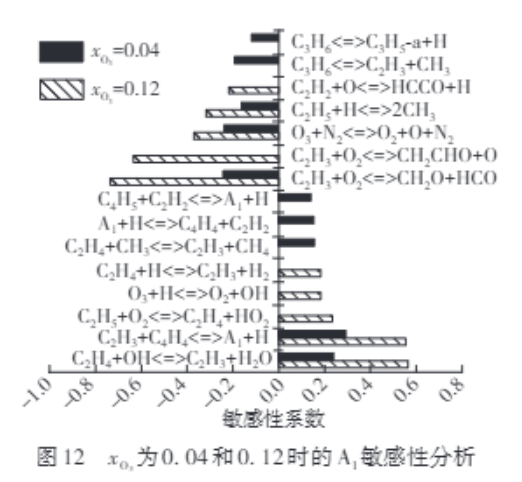
研究O3对生物柴油燃烧过程中多环芳烃(PAH)的影响,可为降低碳烟排放提供新思路。该研究建立了生物柴油替代物基础反应机理与PAH反应机理、O3反应机理耦合的骨架反应机理,用于模拟O3对生物柴油替代物对冲扩散火焰中PAH生成的影响和作用机制。该骨架反应机理共包括138种组分和608个反应。分析发现:O3的添加会使燃料侧形成一个局部快速温升区,随着初始O3摩尔分数的增大,该区域温升速率增大且位置越靠近燃料出口侧,这是燃料被初步氧化释放热量的结果;随着初始O3摩尔分数的增大,PAH的最大摩尔分数先增大后减小;当初始O3摩尔分数增大到0.04时,主要的PAH如苯(A1)、萘(A2)、蒽(A3)和芘(A4)的最大摩尔分数分别是初始O3摩尔分数为0.00时的4.57、6.76、16.16、12.38倍,说明O3的添加对PAH摩尔分数的影响较为显著,并且对A3的影响最大;同时,A1的生成途径发生变化,生成A1的主要反应机制从由C2H2主导转变为由C2H3主导;而当初始O3摩尔分数增大到0.12时,A1、A2、A3、A4的最大摩尔分数分别是初始O3摩尔分数为0.00时的0.880、0.357、0.375、0.143倍,原因在于C2H3自由基会被氧化,从而抑制了A1的生成。
甘云华 , 刘卓龙 , 匡华临 , 韩彦杰 , 李华 . 臭氧对生物柴油替代物燃烧中多环芳烃生成的影响[J]. 华南理工大学学报(自然科学版), 2025 , 53(7) : 11 -20 . DOI: 10.12141/j.issn.1000-565X.240235
Studying the effect of Ozone (O3) on polycyclic aromatic hydrocarbon (PAH) during the combustion process of biodiesel can provide new insights for reducing soot emissions. A skeletal reaction mechanism of biodiesel surrogates coupled with an O3 reaction mechanism and a PAH reaction mechanism was constructed for modeling the effect and mechanism of O3 on PAH formation in a counterflow flame of biodiesel surrogates. The final mechanism consists of 138 species and 608 reactions. Analysis show that the addition of O₃ creates a localized rapid temperature rise zone on the fuel side. As the initial O₃ mole fraction increases, the temperature rise rate in this zone intensifies and its position shifts closer to the fuel outlet, resulting from the preliminary oxidation of the fuel releasing heat. Furthermore, the maximum mole fraction of PAH initially increases and subsequently decreases with increasing initial O₃ mole fraction. When initial O3 mole fraction increases to 0.04, the maximum mole fraction of major PAH such as benzene (A1), naphthalene (A2), anthracene (A3), and pyrene (A4) are 4.57, 6.76, 16.16, 12.38 times that at initial O3 mole fraction of 0.00, respectively. The addition of O3 has a significant impact on the concentration of PAH, and has the greatest impact on A3. At the same time, the pathway of benzene (A1) generation shifts from C₂H₂-dominated to C₂H₃-dominated mechanisms. And when initial O3 mole fraction increases to 0.12, the maximum mole fractions of A1, A2, A3, and A4 are 0.880, 0.357, 0.375, and 0.143 times that at initial O3 mole fraction of 0.00. It is because that the C2H3 radicals are oxidized, thereby inhibiting the production of A1.

| [1] | 梅德清,俞玥,高亚平,等 .多组分燃料液滴高温蒸发耦合机制研究[J].华南理工大学学报(自然科学版),2023,51(3):33-40,82. |
| MEI Deqing, YU Yue, GAO Yaping,et al .Coupling mechanism of high-temperature evaporation of multi-component fuel droplets[J].Journal of South China University of Technology (Natural Science Edition),2023,51(3):33-40,82. | |
| [2] | ISSARIYAKUL T, DALAI A K .Biodiesel from vegetable oils[J].Renewable and Sustainable Energy Reviews,2014,31:446-471. |
| [3] | WESTBROOK C K, NAIK C V, HERBINET O,et al .Detailed chemical kinetic reaction mechanisms for soy and rapeseed biodiesel fuels[J].Combustion and Flame,2011,158(4):742-755. |
| [4] | FISHER E M, PITZ W J, CURRAN H J,et al .Detailed chemical kinetic mechanisms for combustion of oxygenated fuels[J].Proceedings of the Combustion Institute,2000,28(2):1579-1586. |
| [5] | HERBINET O, PITZ W J, WESTBROOK C K .Detailed chemical kinetic mechanism for the oxidation of biodiesel fuels blend surrogate[J].Combustion and Flame,2010,157(5):893-908. |
| [6] | LUO Z, PLOMER M, LU T,et al .A reduced mechanism for biodiesel surrogates for compression ignition engine applications[J].Fuel,2012,99:143-153. |
| [7] | NI Z, LI F, WANG H .Simplification of the combustion mechanism of Jatropha biodiesel surrogate fuel and reaction path analysis[J].Energy,2023,282:128859/1-9. |
| [8] | 王晶,张漫 .不同前驱体模型对航空煤油扩散火焰碳烟颗粒生成的影响研究[J].工程热物理学报,2021,42(12):3286-3295. |
| WANG Jing, ZHANG Man .Study on the influence of different soot inception models on the soot particles formation in a aviation kerosene diffusion flame[J].Journal of Engineering Thermophysics,2021,42(12):3286-3295. | |
| [9] | SLAVINSKAYA N A, FRANK P .A modelling study of aromatic soot precursors formation in laminar methane and ethene flames[J].Combustion and Flame,2009,156(9):1705-1722. |
| [10] | WANG H, DENEYS R R, YAO M F,et al .Development of an n-heptane-n-butanol-PAH mechanism and its application for combustion and soot prediction[J].Combustion and Flame,2013,160(3):504-519. |
| [11] | DONG X, CHANG Y, NIU B,et al .Development of a practical reaction model of polycyclic aromatic hydrocarbon (PAH) formation and oxidation for diesel surrogate fuel[J].Fuel,2020,267:117159/1-14. |
| [12] | WU Y X, LIU F X, SUN Y L,et al .Effects of carbon dioxide and water vapor addition on benzene and PAH formation in a laminar premixed CH4/O2/Ar flame[J].Combustion Science and Technology,2019,191(10):1866-1897. |
| [13] | LIU F, DWORKIN S B, THOMSON M J,et al .Modeling DME addition effects to fuel on PAH and soot in laminar coflow ethylene/air diffusion flames using two PAH mechanisms[J].Combustion Science and Technology,2012,184(7/8/9):966-979. |
| [14] | ZENG W .Single zone simulation of polycyclic aromatic hydrocarbon formation in n-heptane HCCI combustion[J].Advanced Materials Research,2011,236/237/238:525-529. |
| [15] | LI S, GAO J, HUANG C,et al .Development of a reduced n-heptane/toluene/unsaturated furans-PAH reaction mechanism for dual-fuel engine applications[J].Fuel,2022,317:123466/1-15. |
| [16] | WANG H, LI Y, IQBAL Z,et al .A comparison study on the combustion and sooting characteristics of base engine oil and n-dodecane in laminar diffusion flames[J].Applied Thermal Engineering,2019,158:113812/1-6. |
| [17] | LIU X L, WANG H, WEI L X,et al .Development of a reduced toluene reference fuel (TRF)-2,5-dimethylfuran-polycyclic aromatic hydrocarbon (PAH) mechanism for engine applications[J].Combustion and Flame,2016,165:453-465. |
| [18] | YAMADA H, YOSHII M, TEZAKI A .Chemical mechanistic analysis of additive effects in homogeneous charge compression ignition of dimethyl ether[J].Proceedings of the Combustion Institute,2005,30(2):2773-2780. |
| [19] | FOUCHER F, HIGELIN P, MOUNA?M-ROUSSELLE C,et al .Influence of ozone on the combustion of n-heptane in a HCCI engine[J].Proceedings of the Combustion Institute,2013,34(2):3005-3012. |
| [20] | MASURIER J, FOUCHER F, DAYMA G,et al .Investigation of iso-octane combustion in a homogeneous charge compression ignition engine seeded by ozone,nitric oxide and nitrogen dioxide[J].Proceedings of the Combustion Institute,2015,35(3):3125-3132. |
| [21] | SEIGNOUR N, MASURIER J, JOHANSSON B,et al .Ozone-assisted combustion of hydrogen:a comparison with isooctane[J].International Journal of Hydrogen Energy,2019,44(26):13953-13963. |
| [22] | LIU S, LIN Z L, ZHANG H,et al .Impact of ammonia addition on knock resistance and combustion performance in a gasoline engine with high compression ratio[J].Energy,2023,262:125458/1-12. |
| [23] | ZHOU Y, GAN Y H, ZHANG C Y,et al .Numerical study for influence of ozone on the combustion of biodiesel surrogates in a homogeneous charge compression ignition engine[J].Fuel Processing Technology,2022,225:107039/1-12. |
| [24] | SLAVINSKAYA N A, RIEDEL U, DWORKIN S B,et al .Detailed numerical modeling of PAH formation and growth in non-premixed ethylene and ethane flames[J].Combustion and Flame,2012,159(3):979-995. |
| [25] | HALTER F, HIGELIN P, DAGAUT P .Experimental and detailed kinetic modeling study of the effect of ozone on the combustion of methane[J].Energy & Fuels,2011,25:2909-2916. |
| [26] | SARATHY S M, THOMSON M J, PITZ W J,et al .An experimental and kinetic modeling study of methyl decanoate combustion[J].Proceedings of the Combustion Institute,2011,33(1):399-405. |
| [27] | HE Y T, LUO Y, ZHANG Q,et al .Effects of air-side dimethyl ether premixing on combustion characteristics of nonpremixed biodiesel/air counterflow flames[J].Fuel,2022,316:123306/1-11. |
| [28] | INAL F, SENKAN S M .Effects of equivalence ratio on species and soot concentrations in premixed n-heptane flames[J].Combustion and Flame,2002,131(1/2):16-28. |
| [29] | SOLMAZ E, BISETTI F .Flamelet chemistry model for efficient axisymmetric counterflow flame simulations with realistic nozzle geometries and gravitational body force[J].Combustion Theory and Modelling,2020,24(5):926-952. |
/
| 〈 |
|
〉 |